China3D printingNet March 18th, Belgian and German researchers have jointly explored new organisms3D printingTechnology, and in the recently published “MSC-based adipose tissue engineering with variable pore size3D printingThe biocompatibility structure was evaluated in the evaluation of gelatin stents. Through mastectomy or other types of soft tissue trauma, the research team3D printingAnd evaluated the gelatin-based scaffold samples.
Because breast cancer is common and even devastating worldwide, people are conducting continuous research on the diagnosis, treatment and procedures of soft tissue regeneration. However, many of today’s methods have problems, from microsurgery complications to high absorption rates. With the advent of adipose tissue engineering, there is a potential for regeneration through the integration of mesenchymal stromal cells (MSCs) and biomaterials.
In this study, photocrosslinkable gelatin methacrylate (Gel-MA) is the material of choice because of its ability to interact with cells and similar advantages to collagen in the extracellular matrix. The author examined the adipogenic differentiation behavior of bone marrow-derived MSCs in porous 3D scaffolds. They are not “restricted” units, but allow three key activities: spatial expansion, movement and distribution.use3D printingSample, the research team can control the parameters and easily change the 3D file and the generated3D printing.
The team uses squeeze-based3D printingIn the form, the sample holder is changed with a distance between the struts of 400 to 800 µm. The corresponding apertures are 230 ± 24 µm (400), 302 ± 30 µm (500), 348 ± 28 µm (600) and 531 ± 33 µm (800).
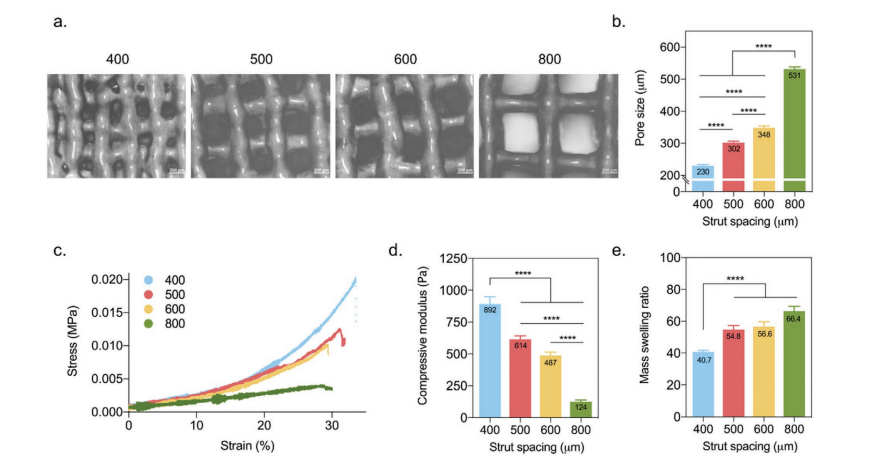
3D printingPhysical and chemical properties of Gel-MA scaffold. a, b) Representative images of holes and hole sizes obtained for different stents. The scale bar represents 200 µm. c, d) The stress-strain curve obtained by the compression test is used to determine the compressive modulus. e) The mass expansion rate of the stent.
During the printing process, maintain control of the high shape fidelity bracket by maintaining the following aspects:
Constant pressure (120 kPa)
Temperature (30°C)
Writing speed (10mm s-1)
The researchers also maintained the consistency of the UV exposure time and used a high-precision nozzle (150 µm) to make stable stents-all stents showed similar pillar widths. Although all samples can absorb water, this ability is improved in samples with larger pores. Overall, the research team pointed out that there is a link between the expansion and mechanical properties of the sample holder, which is due to the increasing mass expansion ratio while the compressive modulus is decreasing.
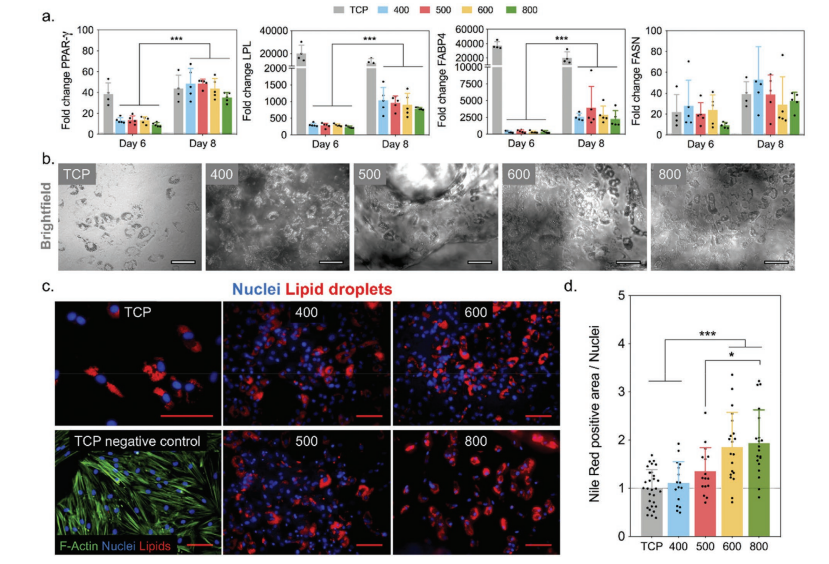
Adipogenic differentiation of MSC on the extruded printing scaffold. a) Compared with scaffolds, MSC uses TCP to express gene expression on adipogenic markers PPAR-γ, LPL, FABP and FASN. b) Brightfield and c) Immunofluorescence images showing clearly visible lipid droplets after 8 days of culture in lipogenic medium. d) Quantify adipogenic differentiation by normalizing the area of Nile Red (stained lipid droplets) to the number of nuclei. Except for the TCP panel in (c) which is 100 µm, the scale bars in all images indicate 200 µm.
The researchers concluded: “Although the stiffness of the extruded Gel-MA in all groups is 3-4 kPa, which mimics the compliance of natural soft tissue, as the pore size increases, the change in compressive modulus reflects the macroscopic view of the scaffold. Structural integrity. In future work, maintaining low stiffness to promote adipogenic differentiation, while improving structural stability to improve implant operability may involve the use of secondary particles or phases to enhance the ink.
We found that in all scaffolds with pore diameters (200-600 µm), MSCs can differentiate into lipid lineages well. However, the spatial distribution and cell infiltration are different, so scaffolds with larger pore sizes (> 500 µm) support simultaneous differentiation and infiltration. These findings indicate that it is important to consider design parameters (such as pore size) when designing a 3D soft tissue regeneration scaffold. “
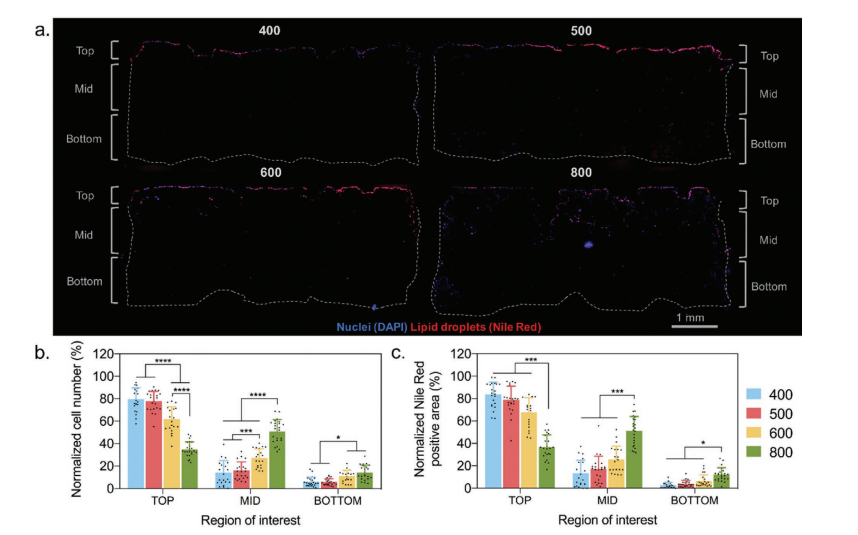
The spatial distribution of cells and lipid droplets in the scaffold. a) On each type of scaffold, representative scaffold cross-sections stained with DAPI (nucleus) and Nile red (lipid drop) show differences in the spatial distribution of cell infiltration and adipogenic differentiation. Quantification of b) cells and c) Nile Red positive areas in different areas of the scaffold.
China3D printingOnline comment: The research on bioprinting and scaffolding is expanding. Although the research on breast cancer and other diseases is crucial, other scientists have published information about seeding dermal fibroblasts to promote cartilage growth.3D printingTo replace the use of bone and various other ongoing research.Maybe in the future3D printingThe organ is no longer a dream.
(Editor in charge: admin)


0 Comments for “3D printed scaffold for tissue resection and tissue regeneration after tissue injury”