
Article Introduction
Due to congenital malformations or coronary artery occlusion,Blood vesselAtresia is usually recanalized via a catheter orsurgicalVascular anastomosis treatment. However, the cell’s response to vascular anastomosis or recanalization is largely unknown, and current techniques rely on restoration rather than optimizing the flow into the occluded artery.
An in-depth understanding of the cellular response after anastomosis may effectively reduce the phenomenon of vascular restenosis. This article constructs an in vitro model to simulate pulmonary artery (PA) anastomosis and procedure planning to reduce vascular restenosis.The bifurcated PA is carried out in a 3D hydrogel structurebiologyPrint to simulate the reconstructed intervascular connection. The PA model was inoculated with human endothelial cells and perfused at a physiological flow rate to form endothelium.
Particle image velocimetry and computational fluid dynamics models show close agreement in quantifying the flow velocity and wall shear stress in bioprinted arteries. These data can be used to identify the highest value of shear stress changes and areas that are prone to narrowing.
Vessel geometry and hemodynamics significantly affect endothelial cell viability, proliferation, arrangement, microcapillary formation and metabolic biological characteristics.This integrated in vitro-in silico method establishes a unique platform to study complex cardiovascular diseases and can directly improve blood flow disorders.Operationplannedclinicaltreatment.
Main experimental results
biology3D printingPA in vitro model:The researchers used a DLP printer and used 20% GelMA to print a bifurcated anastomosed PA atresia model, including 1 unoccluded blood vessel, 1 occluded blood vessel, and 1 catheter anastomosed to the blood vessel. The tissue stiffness range printed in this study is relatively higher than the reported stiffness of natural non-diseased PA (4-20 kPa), but it is consistent with the reported stiffness of various diseased PA models (50-100 kPa).
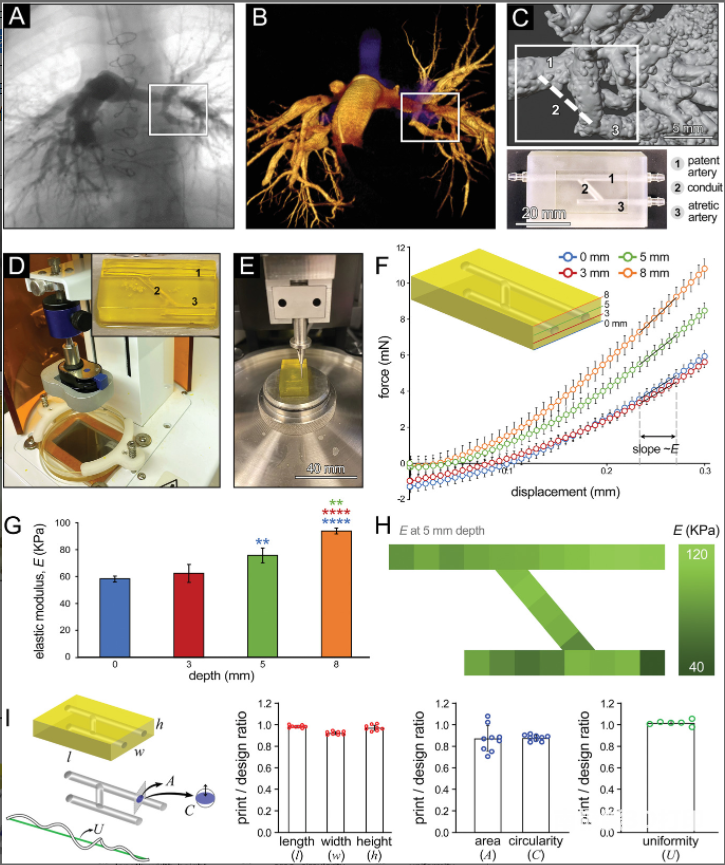
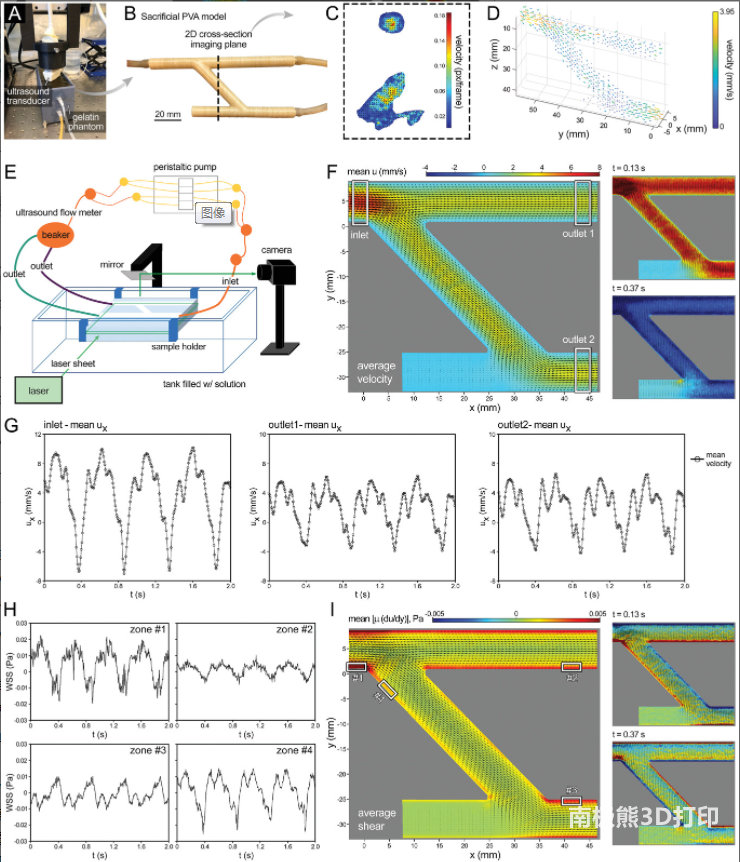
Flow hemodynamics of PA structure:The combination of PIV flow visualization technology and CFD modeling tools provides a powerful method to analyze and quantify the blood (or medium) perfusion within the established lung connections in the bioprinted PA geometry. Overall, the CFD results have acceptable consistency with speed and WSS PIV measurements and calculations, highlighting the importance of CFD simulation for effectively predicting and evaluating the impact of design parameters on flow performance. The selective CFD study in this work shows how to develop a personalized surgical planning platform, guided by in vitro modeling, and through computational optimization methods to effectively explore various anastomotic scenarios and determine the best postoperative flow characteristics.
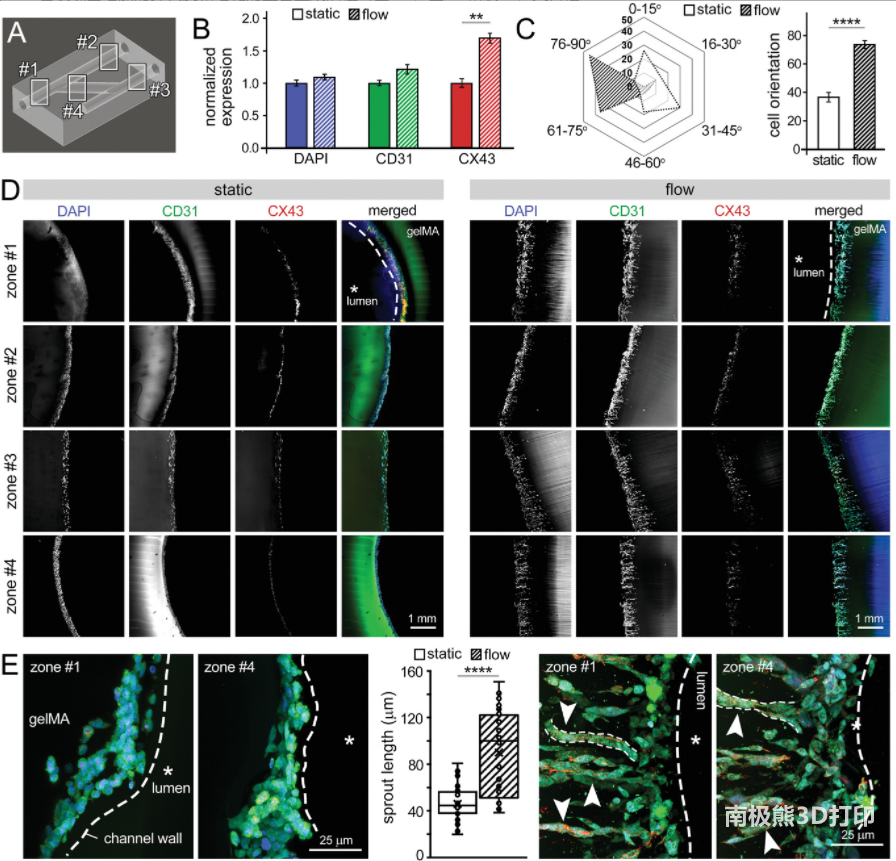
Biological characteristics of PA model:The results of bioanalysis showed that HUVEC in PAA constructs cultured under flow conditions showed a more efficient metabolism than under static conditions. These results indicate that the inclusion of continuous flow provides a more physiologically relevant microenvironment that encourages cells to undergo cell growth while maintaining a stable metabolism. It also shows that properly designed engineered tissues can generalize the physiological complexity of tissues/organs in the body. In future work, this analysis can be very effective in designing and developing feeding strategies that maintain an ideal metabolic environment.
Article summary
The multi-purpose perfusion vascularized tissue platform based on the most advanced 3D bioprinting and bioreactor technology can be used for modeling and treatment planning of complex vascular atresia. In this model, we incorporate human endothelial cells into the bioprinting design to achieve simplified cell-based vascular simulation. These models can also be used for large-scale and reliable bioprinting for statistically significant multi-dimensional analysis. In addition, the vascular mimic can be easily perfused with a bionic flow rate. We hope that our findings here can promote a positive transformational impact on the treatment of vascular stenosis in the development of surgical and transcatheter interventions, drug treatments, and mapping cell interactions. In particular, this model can provide an important reference for the postoperative influence of the vascular function and blood flow changes in the affected vasculature bifurcation and trigeminal areas.
references
Tomov, ML, Perez,L., Ning, L., Chen, H., Jing, B., Mingee, A., Ibrahim,S., Theus, AS, Kabboul, G., Do, K., Bhamidipati, SR, Fischbach, J., McCoy, K., Zambrano, BA, Zhang, J., Avazmohammadi, R., Mantalaris, A., Lindsey, BD, Frakes, D., Dasi, LP, Serpooshan, V., Bauser -Heaton,H., A 3D Bioprinted In Vitro Model of Pulmonary Artery Atresia to EvaluateEndothelial Cell Response to Microenvironment. Adv. Healthcare Mater. 2021,2100968. https://doi.org/10.1002/adhm.202100968
(Editor in charge: admin)


0 Comments for “AHM | 3D printed pulmonary artery atresia model for endothelial cell response simulation, effectively reducing vascular restenosis”