China3D printingNet June 17th, Belgium3D printingSoftware company Materialize has received FDA clearance to market Mimics Enlight, a 3D modeling software for planning cardiovascular surgery.
Bryan Crutchfield, vice president and general manager of Materialise North America, said: “We believe our mission is to create a better, healthier world to contribute, and we work closely with teams at partner hospitals and medical device companies to plan3D printingin order to improve the execution of their programs.
With FDA clarity on molds, we are expanding our 3D toolkit for treating patients with complex cardiovascular problems and cardiac patients with MITRAL valve replacement. “
Mimics Enlight was developed in partnership with Henry Ford Health System, a Detroit healthcare organization founded by American industrialist Henry Ford. This software is specifically designed for planning complex transcatheter mitral valve replacement (TMVR) procedures.
The mitral valve is located between the left atrium and left ventricle of the heart. Among several types of mitral valve disease, mitral regurgitation is reported to be the most common in developed countries, with approximately 10% of people over the age of 75 suffering from the disease.
While mitral valve repair or replacement is the best way to treat the condition, patients don’t often undergo surgery. This is due to the risks of surgery.
Over the years, the company has manufactured devices for minimally invasive transcatheter mitral valve repair treatments. These installations include the MitraClip at Abbott Vascular in California and the Carillon at Cardiac Dimensions in Washington. But even if these devices are available for implantation, performing the surgery remains a formidable challenge.
Mimics Enlight is designed to assist in surgical planning for TMVR. The software can design patient-specific 3D models that can be studied to accurately implant surgical devices. For efficient planning of cardiac surgery, Mimics Enlight also generates reports and workflows.
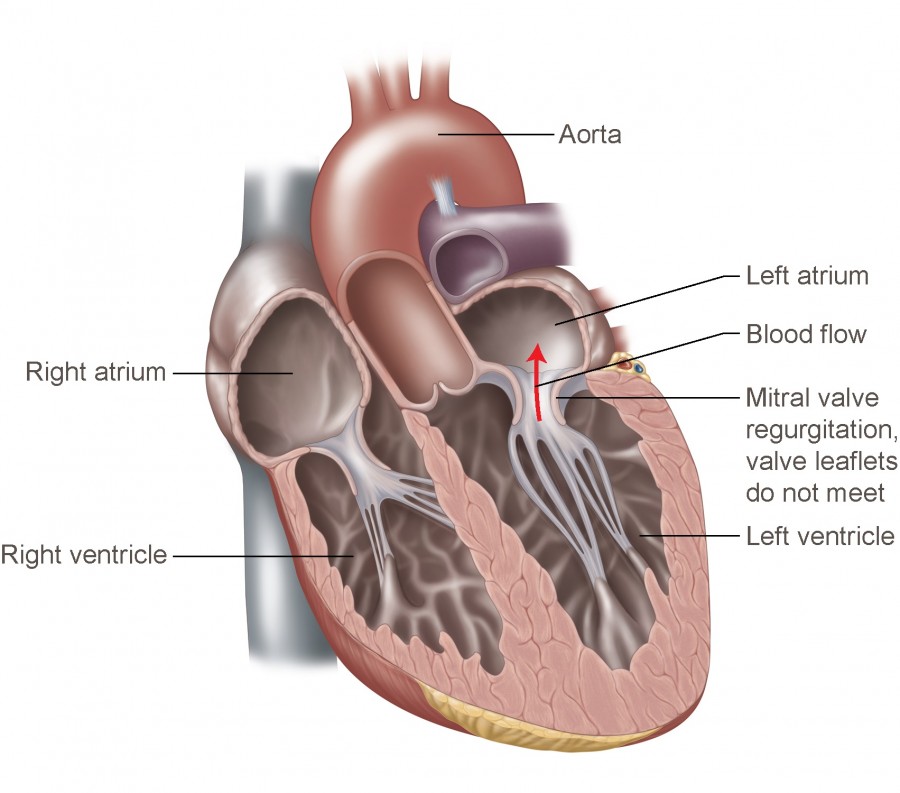
Mitral valve regurgitation diagram
Software has been considered a medical device since the FDA revised the guidelines. Therefore, 3D modeling software for planning and performing surgery requires FDA approval.As such, Materialise’s Mimics Innovation Suite is the first FDA-approved3D printingsoftware.Since then, Materialise has also verified3D printingMachines, such as the Ultimaker S5 and Stratasys J750 and J735, are used in the Mimics Innovation Suite.On the FDA approval of Mimics Enlight, Brigitte de Vet-Veithen, Vice President of Materialise Medical, said: “Materialise has a wealth of medical technology and experience built over 20 years in the development and implementation of Mimics’ innovative suite. We provide patient-specific solutions. Protocol expertise is the foundation of Mimics Enlight Mitral’s ability to continuously view and measure the complex mitral valve anatomy of each patient.”
China3D printingNet compiled from:3dprintingindustry.com
(responsible editor: admin)


0 Comments for “Belgian 3D printing software company receives FDA approval for mitral valve surgery software”