Globally, more than 5 million people are affected by corneal blindness and limbal stem cell defect (LSCD), which is a common cause.Traditional LSCD treatment uses
Operation
Repair interventions, using sources such as amniotic membrane (AM) as a substrate or stent, combined with limbal autografts or allografts.These treatments are limited by the lack of
standard
The production of chemical AM, the risk of iatrogenic LSCD and immune rejection.
Recently, the Shaochen Chen team from the University of California, San Diego and the Sophie X. Deng team from the University of California, Los Angeles have collaborated to use methacryloyl gelatin (GelMA) or hyaluronic acid glycidyl methacrylate (HAGM) based on DLP.
biology
The micro hydrogel scaffold produced by printing can support the viability of the encapsulated primary rabbit LSC (rbLSC) in culture.The results of the study are the regeneration of stem cell therapy and corneal reconstruction
medicine
Provides valuable insights. The related paper “Bioprinting of Dual ECM Scaffolds Encapsulating Limbal Stem/Progenitor Cells in Active and Quiescent Statuses” was published in the journal Biofabrication.
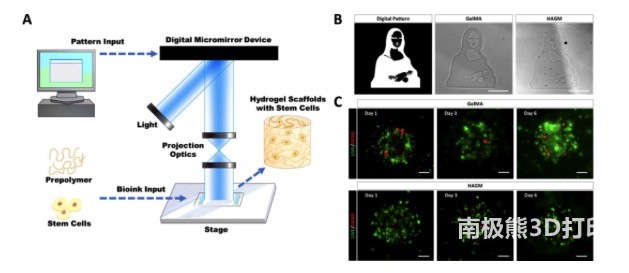
As shown in Figure 1, the researcher’s customized DLP-based bioprinting system can manipulate light in space according to user-defined input design, so that the cellular hydrogel structure containing different material components can be accurately patterned based on photopolymerization. change. Using DLP-based bioprinting technology, GelMA or HAGM-based hydrogel scaffolds with complex patterns and micro-scale resolution can be manufactured in a few seconds. In order to test the biocompatibility, a gelMA or HAGM-based bioprinting scaffold was made to encapsulate the primary rbLSC. Live-dead staining confirmed that both types of bioprinted scaffolds can support the viability of encapsulated rbLSCs after 7 days of culture. In short, a DLP-based bioprinting system can be used to manufacture GelMA and HAGM scaffolds that encapsulate viable primary rbLSC.
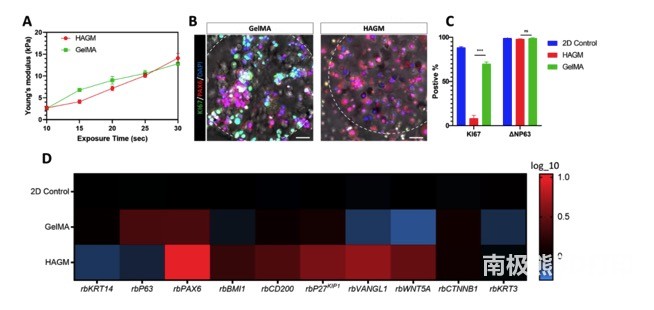
As shown in Figure 2, the mechanical test of the bioprinting scaffold based on GelMA and HAGM shows that there is a positive linear correlation between the Young’s modulus and the exposure time in the printing system. GelMA and HAGM scaffolds have similar Young’s modulus, and the exposure time is set to 25 seconds as the main bioprinting parameter for subsequent experiments. Immunofluorescence staining showed that PAX6 was expressed in GelMA and HAGM-encapsulated rbLSC, while the expression of proliferation marker KI67 only existed in GelMA-based scaffolds. Compared with those encapsulated in GelMA, flow cytometry identified a significant decrease in the percentage of KI67-positive rbLSC encapsulated in HAGM-based bioprinting scaffolds, while the positive ratio of dryness markers ΔNP63 remained the same in both scaffolds . The researchers also performed transcription analysis using real-time qPCR to compare rbLSCs in 2D culture or packaging with GelMA or HAGM-based bioprinted scaffolds. Collectively, these results demonstrate the active state of rbLSCs encapsulated in GelMA-based stents and the resting properties of rbLSCs in HAGM-based stents.
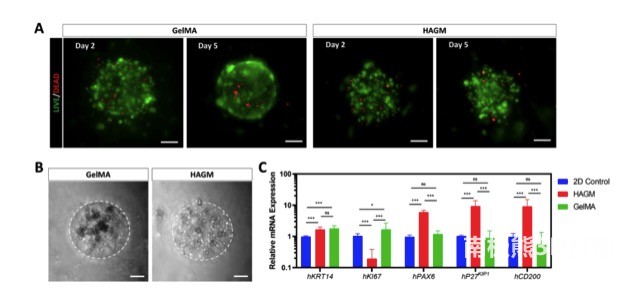
As shown in Figure 3, the researchers further explored the LSC-ECM interaction in human LSCs. Similar to rbLSCs, live-dead staining showed that most encapsulated hLSCs are still viable in both types of bioprinting scaffolds during culture. . Consistent with rbLSCs, the aggregate colonies of hLSCs mainly exist in GelMA-based scaffolds, but are rarely observed in HAGM-based scaffolds. Real-time qPCR showed that hLSCs encapsulated in HAGM-based scaffolds had significantly higher expressions of PAX6, CD200, and P27KIP1, while the expression of KI67 was significantly down-regulated compared with the two-dimensional control and GelMA groups.These results reinforce the observations of the different responses of LSCs to the surrounding ECM components, and they seem to be consistent regardless of whether LSCs are isolated from rabbits or humans.
clinical
Research can be very valuable.
As shown in Figure 4, the researchers first printed a micro-scale “yin and yang” pattern with a mixture of GelMA and HAGM and fluorescent microspheres. In the subsequent printing, the fluorescent microspheres were replaced with primary rbLSCs, and the status of the encapsulated rbLSCs in different parts of the dual ECM model was verified. Therefore, through this bioprinting method, a dual ECM “yin and yang” model can be manufactured, and its separate ECM part induces the active/static state of LSC.
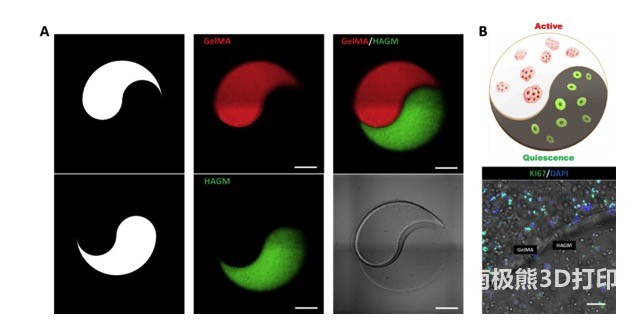
In summary, this research uses DLP-based bioprinting to manufacture GelMA and HAGM-based engineered micro-hydrogel scaffolds. These scaffolds not only support the viability of the encapsulated primary rbLSCs and hLSCs, but also exhibit differential regulation. It was found that LSCs exhibit an ECM-dependent activity/quiescence state because they actively proliferate in GelMA-based stents and exhibit quiescent characteristics in HAGM-based stents. Based on these findings, a bioprinted dual ECM “yin and yang” model containing active and static LSC was created. Together, these results illustrate an innovative engineering method for disease modeling, drug screening, and development of LSC-based regenerative therapies to treat LSCD and related ocular diseases.
(Editor in charge: admin)


0 Comments for “Biofabrication: DLP printing containing stem cells for corneal reconstruction treatment”