The Cleveland Clinic, a well-known American multi-professional academic medical center, announced that a customized airway stent developed by a team of clinicians and engineers has been approved by the FDA and will be commercialized by the clinic’s subsidiaries.In the manufacturing process of this customized bracket, a customized3D printingMold.
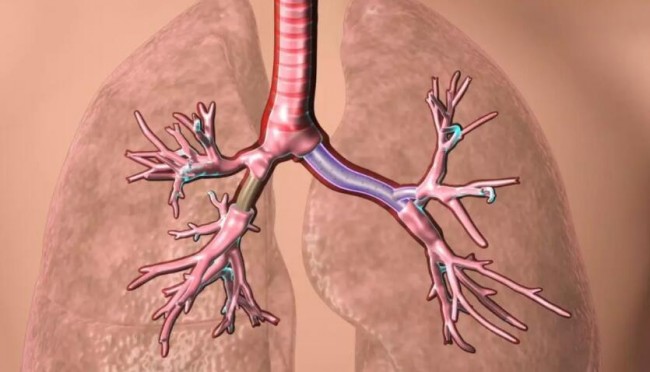
Schematic diagram of customized tracheal stent implantation. Source: Cleveland Clinic
Approved tracheal stents are used to treat severe breathing disorders (such as those caused by tumors, inflammation, trauma, or other masses) to keep the patient’s airway open. Before obtaining the medical device registration certificate, this type of tracheal stent has been used in clinical treatment, and is only used to treat patients whose other treatment methods have failed.
Standardized airway stents are limited in size and shape, and are usually used for larger airways. However, the anatomy of each patient is unique, and standardized products are difficult to match perfectly, especially for patients with complex conditions. If the stent is improperly installed, it will cause kinking and bending of the stent and airway complications, such as the growth of new tissue, mucus impingement, and tissue death.
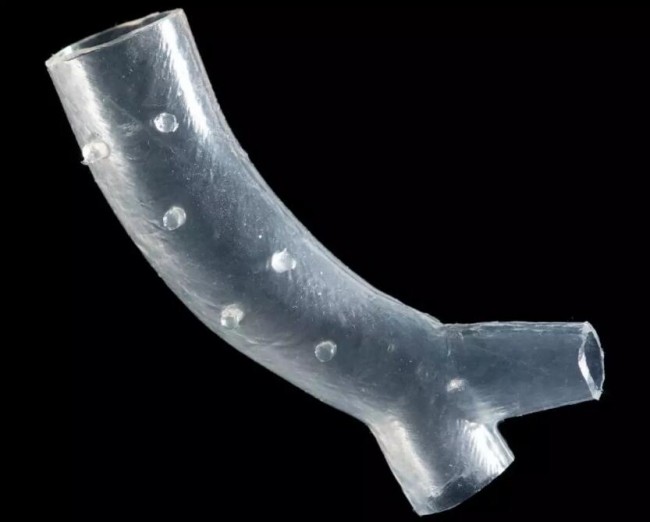
pass through3D printingTrachea bracket for mold manufacturing. Source: Cleveland Clinic
To solve these problems, the doctors and engineering team of the Cleveland Clinic used medical imaging technology and 3D visualization software as well as3D printingTechnology to develop customized tracheal stents that are fully adapted to the patient’s anatomy.3D printingThe technology is used to manufacture custom-made tracheal stent molds. After the mold is manufactured, it will be used for tracheal stent molding. The material of the tracheal stent is medical grade silicone.
Traditional stents may need to be replaced or cleaned frequently due to improper installation problems, and because they are matched to the patient’s anatomy, customized tracheal stents are more tolerant than standardized products. According to research conducted by the Cleveland Clinic, custom-made stents last for about one year on average, compared to 90 days for ordinary stents.
Although the customized tracheal stent developed by the Cleveland Clinic is not used3D printingTechnology is directly manufactured, but each bracket needs a matching3D printingMold.This application is similar to the manufacture of dental invisible orthodontics, although orthodontics are not directly used3D printingProduced, but each orthosis needs a corresponding3D printingDental mold.
Each patient is a unique individual. It is undoubtedly an ideal choice to provide patients with personalized dentures or implants and other medical devices, but it has long been limited by the economics of personalized product manufacturing or medical approval procedures. The reason is that standardized medical devices are still the mainstream.From the perspective of 3D Science Valley, with3D printingThis highly advantageous technology in mass-customized production has matured, and the development of more personalized devices has become possible.
Customized medical devices have also been applied and developed in my country, and the regulations on the supervision and management of customized medical devices have been officially implemented on January 1, 2020.
(Editor in charge: admin)


0 Comments for “Commercialized 3D printed medical devices have added new members! FDA approved a customized tracheal stent”