Skeletal muscles play a vital role in human movement and other dynamic activities.In order to treat critical volumetric muscle defects, existing studies use autologous muscle tissue
Implants
with
Operation
Remove necrotic or damaged muscle tissue to enhance healing potential.However, because skeletal muscle has a kind of muscle fiber bundle and
Blood vessel
The parallel arrangement of the structure, due to the limited controllability of muscle fiber structure and related blood vessel formation, leads to low regeneration efficiency, and traditional treatments are often ineffective.
For this reason, GeunHyung Kim’s research group from Sungkyunkwan University proposed an electrofluidic direct-write cell fiber technology, which can directly print loaded living cells in oriented micro-scale fibers, and induce myoblasts to grow along the printing direction. Related papers :Electrohydrodynamic-direct-printed cell-laden microfibrous structure using alginate-based bioink for effective myotube formation was published in Carbohydrate Polymers.
This study demonstrates an alginate-based
biology
Ink and new biological manufacturing methods to obtain a mechanically stable and orientable microfibrous cell-carrying composite structure can effectively induce skeletal muscle tissue engineering for chip muscle or restore bulk muscle defect structures.
First, the researchers designed a nozzle with an auxiliary electrode, which has a more evenly distributed electric field than a single tip. The printing material is composed of alginate/PEO/fibrin bio-ink which is carried by C2C12 on myoblasts, and the cell-carrying hydrogel is sprayed and deposited on the grounding plate under suitable electric direct writing printing conditions to form the designed cell-carrying The microfiber structure is shown in Figure 1.
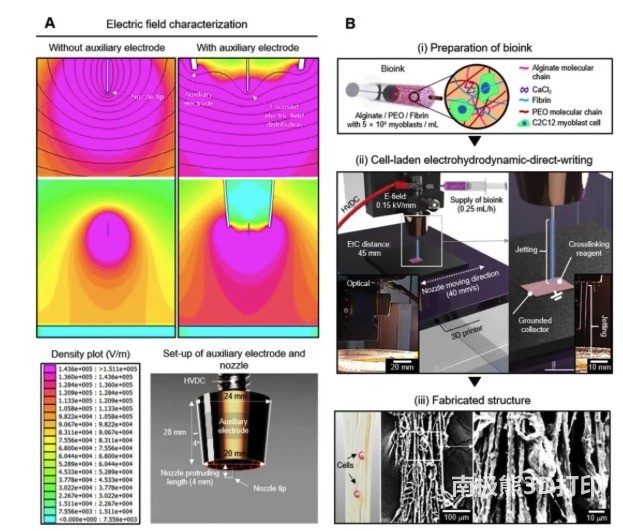
In order to perform electrical direct writing printing smoothly, the researchers evaluated the different components of the bio-ink, and finally determined the appropriate composition ratio, the fiber state is stable and the diameter is within 20μm, as shown in Figure 2.
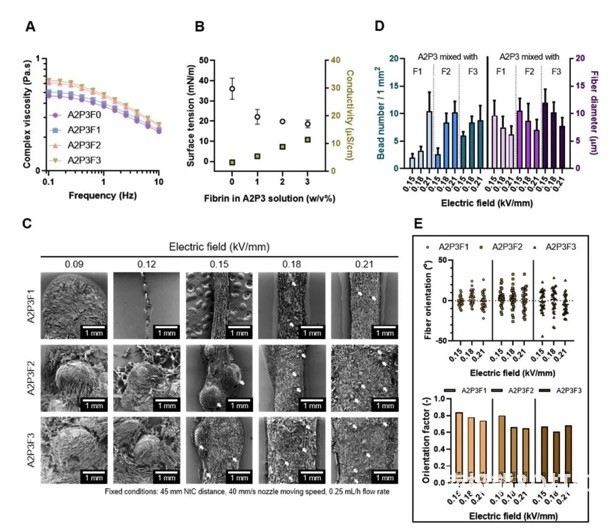
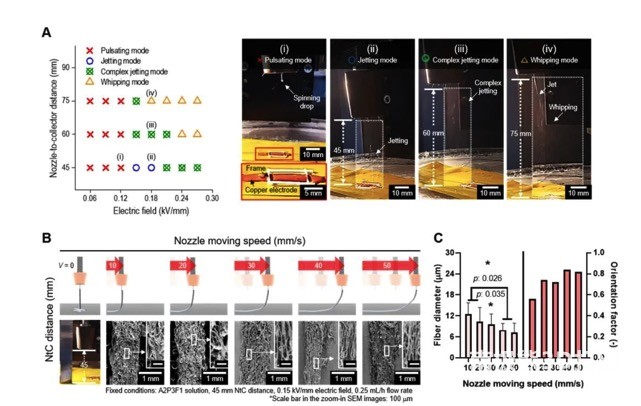
The researchers further explored suitable printing parameters, including the applied electric field, nozzle distance, and printing speed. The effect of each parameter is shown in Figure 3. Finally, the electric field strength is 0.15kV/mm, the nozzle distance is 45mm, and the printing speed is 40mm/s to prepare the cell-carrying microfiber scaffold.
In order to compare the electro-direct write-carrying cell fiber and the traditional cell electrospinning, the researchers tested the cell viability of the bio-ink under different electric field strengths, as shown in Figure 4. According to the experimental results, the electric field of 0.15 kV/mm can keep the cell survival rate high, and the fiber formation is better, so this parameter is selected to prepare the uniaxially arranged cell-carrying microfiber structure. Compared with traditional cell electrospinning, electric direct writing can better deposit in designated parts and reduce loss, and due to dense fiber deposition, the number of cells in electric direct writing fibers is significantly higher than that of traditional cell electrospinning. In addition, in terms of cell viability and cell proliferation rate, electric direct writing fibers are better than cell electrospinning, and their mechanical properties are closer to natural muscle characteristics.
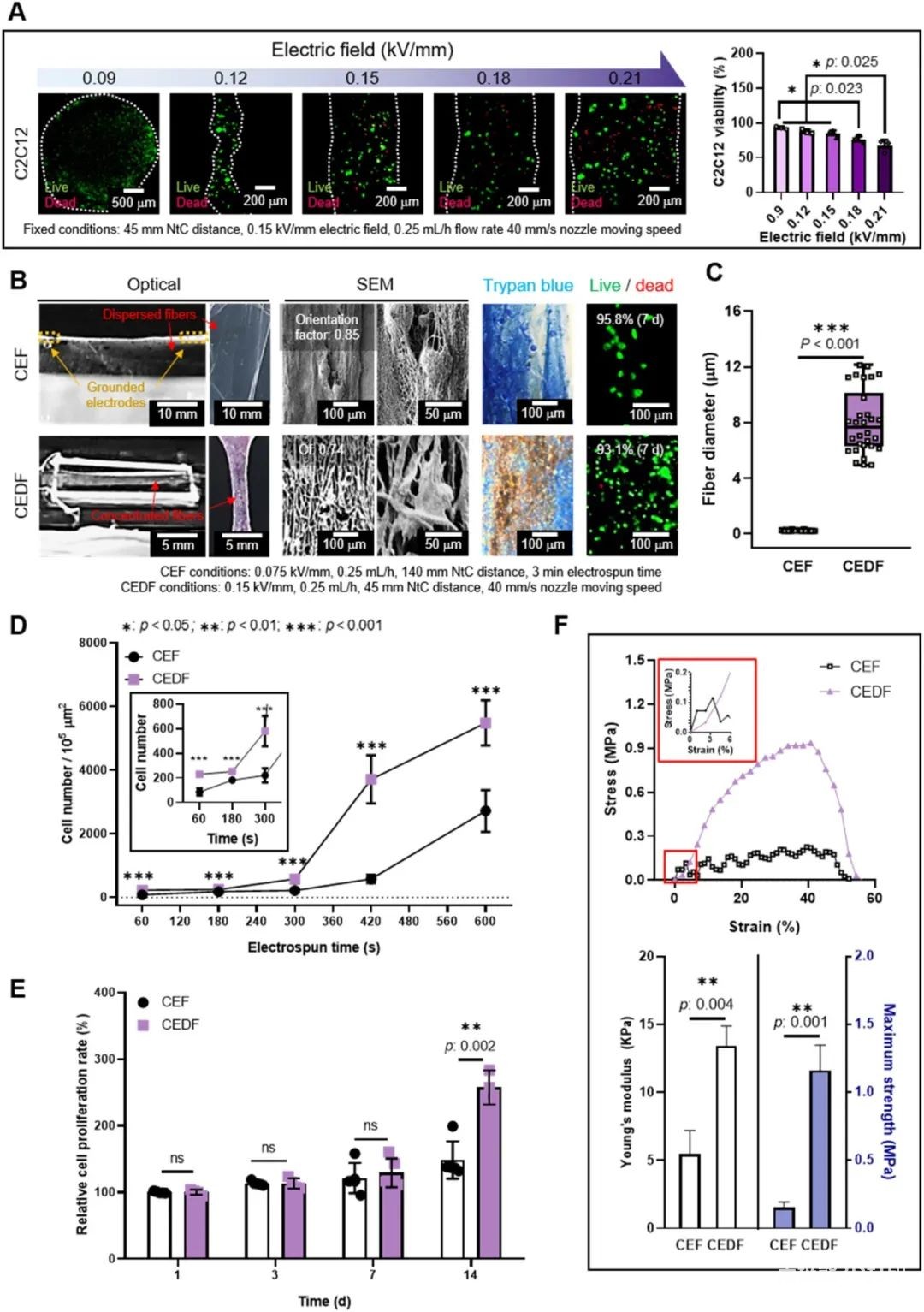
The researchers further compared the traditional extrusion biological3D printing, The in vitro cell characterization of traditional cell electrospinning and electric direct writing of cell fibers. The results are shown in Figure 5.Extrusion Biology3D printingThe morphology of the myosin heavy chain (MHC) on the fiber is random; while the traditional cell electrospinning and electrical direct writing cell fibers show a directional arrangement due to the ultrafine fiber morphology. However, the arranged cells only cover the surface of the traditional electrospinning group, and the cells are connected to each other in bundles on the electric direct writing fibers. This effective cell arrangement can simultaneously have high cell density and appropriate mechanical properties, as well as orientation properties. Other quantitative results also indicate that the electrical direct writing of cell fibers is beneficial to the arrangement and differentiation of myoblasts, and promotes myogenic differentiation and maturation of myotubes. Therefore, the researchers used electrical direct-write cell fibers to co-culture C2C12 myoblasts and HUVECs.
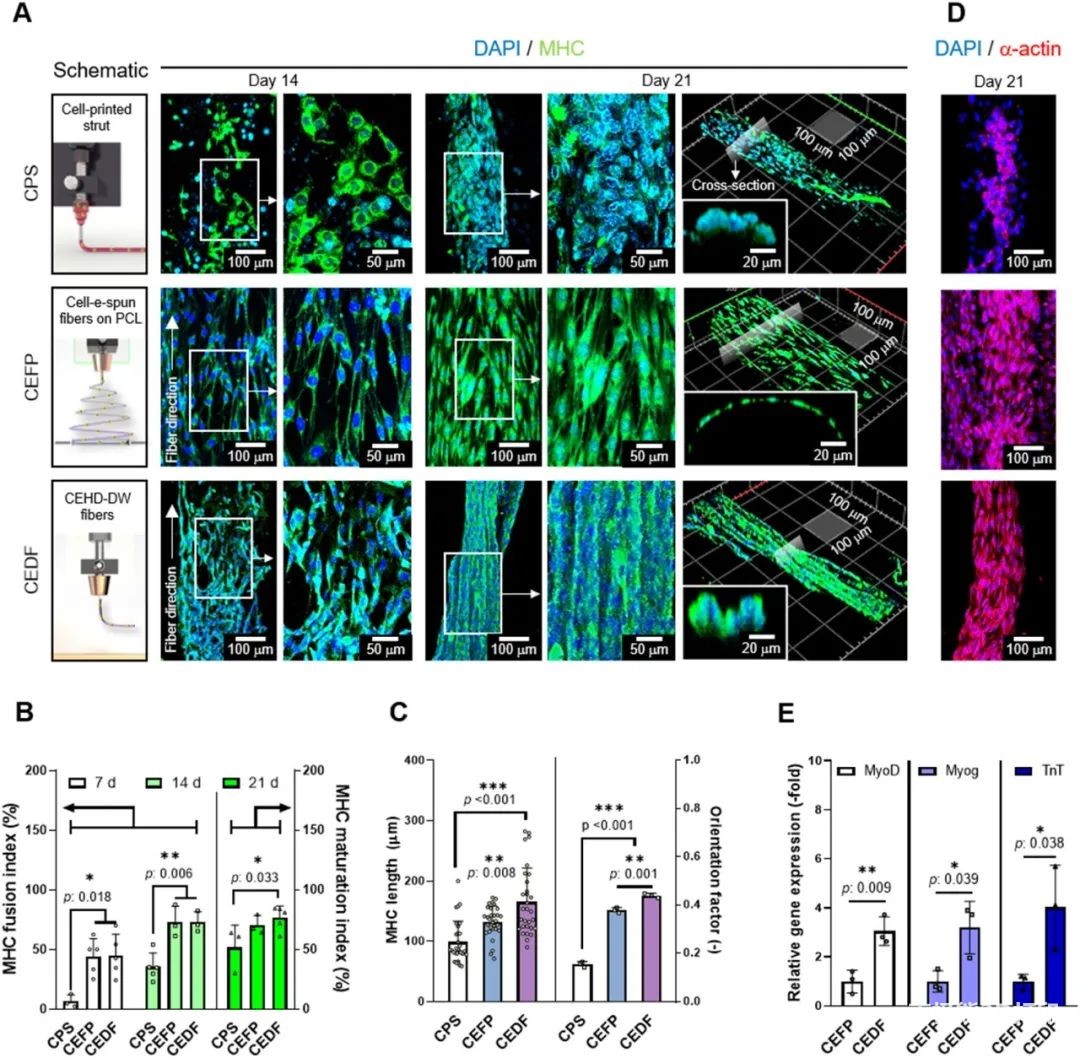
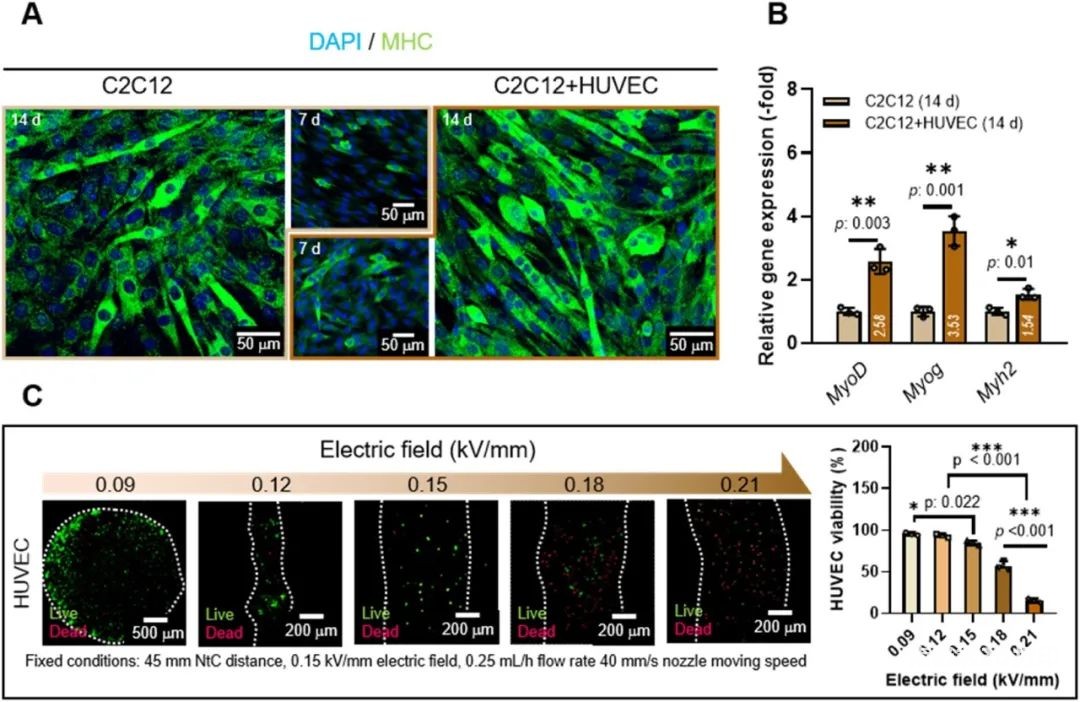
Next, the researchers studied the effect of the loading structure of HUVECs on the myogenic activity of the co-culture structure, Figure 6. The results confirmed that the co-culture of C2C12 and HUVECs is beneficial to muscle tissue regeneration. Before using C2C12 and HUVECs to prepare the composite fiber structure, the cell viability and fiber morphology (0.09-0.21 kV/mm) of HUVECs under the electric direct writing process were tested. According to the results, the parameters of 0.15 kV/mm were finally selected to make the carrier. The fiber structure of HUVECs.
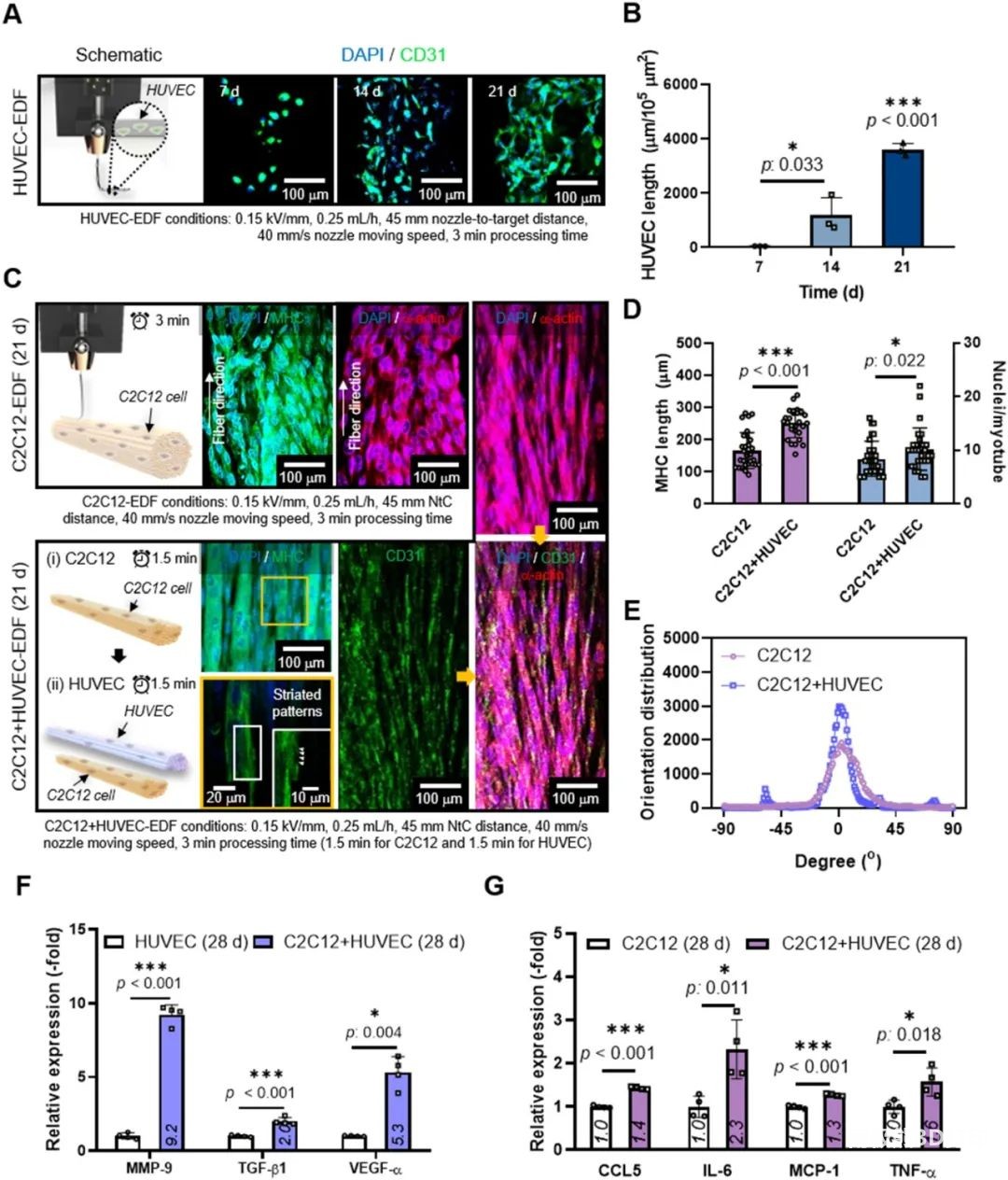
As shown in Figure 7(a), the HUVEC-EDF loaded with HUVECs was prepared, as shown in Figure 7(A), which shows that the preparation process and microenvironment can support the growth of HUVECs. Then, C2C12-loaded fibers (C2C12-EDF) and C2C12/HUVECs-loaded composite fibers (CH-EDF) were prepared, and the in vitro cell viability was compared, Figure 7(C). According to the analysis of the expression detection results of related cytokines, due to the synergy of biophysical and biochemical signals, the structure of the microfiber complex loaded with C2C12/HUVECs can efficiently regenerate muscle tissue.
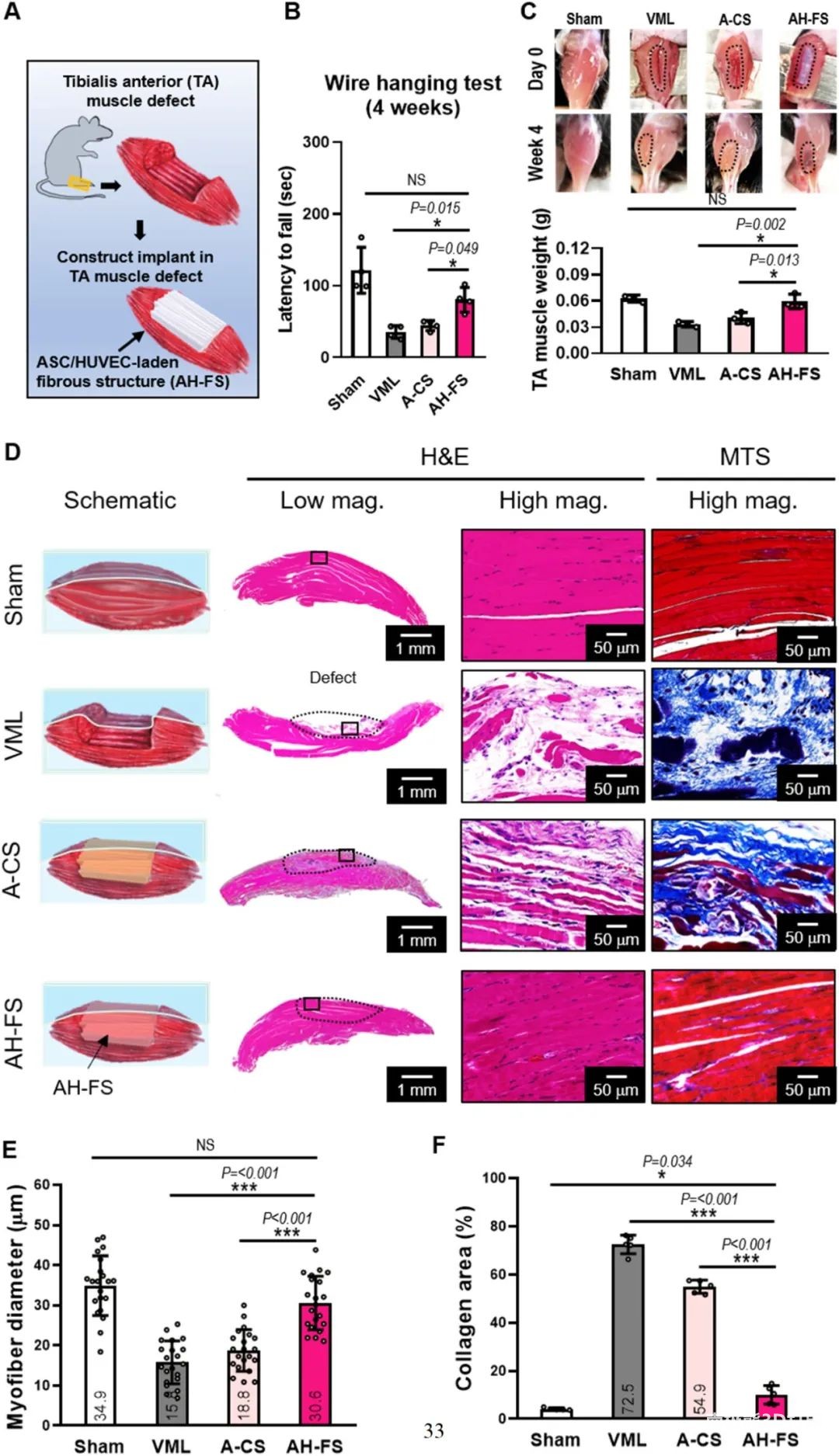
Finally, in order to evaluate the regenerative ability of muscle defects, as shown in Figure 8, the electrical direct writing cell-carrying fiber technology was used to construct muscle structure (AH-FS) with adipose stem cells (ASCs) and HUVECs.Use GelMA and3D printingThe process is used as a control to manufacture a conventional structure (A-CS) with ASC. After comparison, the AH-FS group significantly induced muscle regeneration. These in vivo results represent the potential of cell-loaded fiber structure to treat volumetric muscle loss (VML).
In conclusion, this research proposes a new process of electro-direct writing cell-carrying hydrogel fibers, which can produce highly oriented cell-containing microfibers. Different from the cell electrospinning process, this method provides a microfiber structure with reasonable mechanical properties and higher cell density, and successfully achieves significant cell growth and myogenic differentiation. This research reveals the possibility of a new type of electrohydrodynamic printing, creating a directional effect of cell-loaded microfiber structures for muscle tissue regeneration.
(Editor in charge: admin)


0 Comments for “Electrofluidic direct-write cell fiber technology, which directly prints loaded living cells in oriented micro-scale fibers”