Vascular transplantation, especially the replacement of small diameter blood vessels (<6mm), is still a major challenge in clinical medicine. Because the implant will cause problems such as blockage, intimal hyperplasia or thrombosis. In order to promote tissue regeneration, vascular stents should also guide cell differentiation to obtain the functions of natural blood vessels. For this reason, the stent must guide the formation of a monolayer of endothelial cells inside the lumen to mimic the vascular intima. In addition, the accumulation and orientation of vascular smooth muscle cells (vSMCs) should be realized to mimic the outer layer of the membrane medium. So far, how to design and manufacture stents that meet the requirements to promote is still a challenge. For this reason, Debby Gawlitta of Utrecht University and Jürgen Groll of Würzburg University published "Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts" on Advanced Functional Materials. A hybrid manufacturing method combining electrospinning and melting near-field electrical direct writing has prepared a double-layer composite tubular scaffold that can simulate tissue structure, which includes a dense fiber network with an inner layer of random orientation and a fiber with controllable outer layer orientation. . The scaffold can induce cells to form a continuous luminal monolayer endothelial cells and a directed smooth muscle cell layer, thereby promoting the specific differentiation of tissue cells. Moreover, this method does not require additional soluble factors or bioactive materials on the surface of the scaffold, indicating that the structural design of the special-shaped scaffold can induce cell growth and differentiation.
There are 5 requirements for vascular stents:
- The scaffold should provide a substrate on which endothelial colony forming cells (ECFCs) form a monolayer.
- The endothelial cell monolayer formed on the scaffold should express mature EC markers, basement membrane components and ECM, as well as signals related to communication with vSMCs.
- The scaffold should have a porous outer layer in which vSMCs can migrate and fill in order to achieve the elongation of the multilayered cell tissue.
- The vSMCs should be arranged in a near-circumferential direction, which is controlled by the direction of the scaffold fibers.
- The scaffold should provide an environment for the differentiation of mesenchymal stem cells (MSCs) into the contraction phenotype of vSMCs
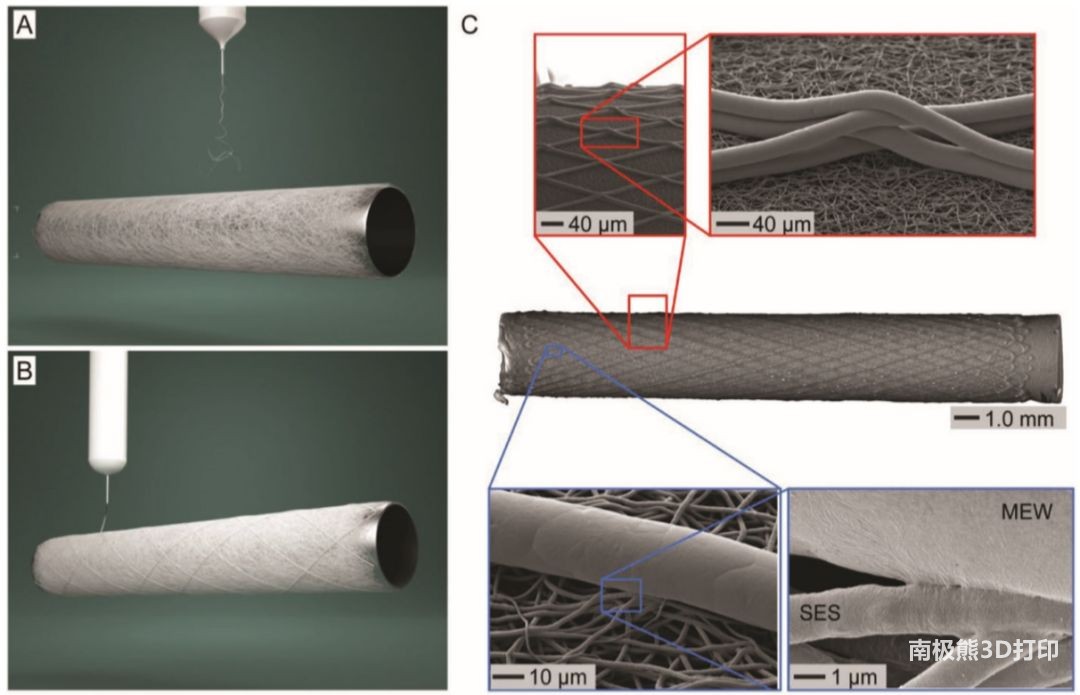
Therefore, the researchers proposed a hybrid vascular stent manufacturing process, as shown in Figure 1. Firstly, PCL fibers were electrospun from the solution to form the inner layer of the tubular scaffold (porosity 80%±5%, inner diameter 3mm), the fiber diameter was 1.4±0.2μm, and the fiber direction was randomly distributed. Secondly, the outer layer is made of the same material using the process of melting near-field electrical direct writing. The fiber diameter is 15.2±4.8μm, the fiber winding angle is between 30° and 70°, and it has a large, controllable aperture.
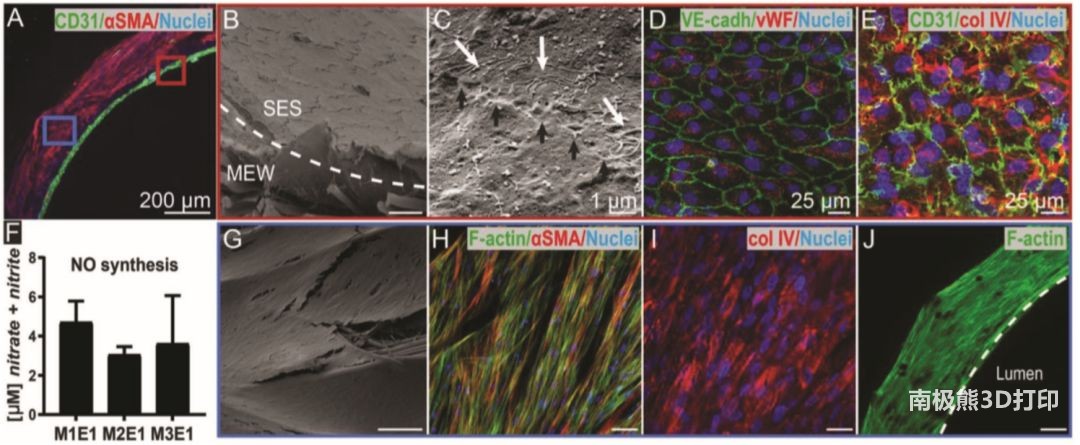
The results of the in vitro experiment of the scaffold are shown in Figure 2. The ECFCs that have not yet been endothelialized are successfully co-cultured with MSCs, and the ECFCs have not penetrated the spinning layer of the solution with very small pore size. Scanning electron microscopy (SEM) showed that a connection was established between adjacent ECs (black arrows) and fibers (white arrows), indicating that endothelial cells are restricted and have low permeability. In the body, endothelial cells with barrier function are the key to resist thrombosis. This creates an inner layer with anticoagulant properties, similar to those of natural endothelium.
Similarly, the monolayer membrane showed positive staining for platelet adhesion glycoprotein factor (vwF) endothelial markers (Figure 2D) and was supported by type IV collagen positive matrix (Figure 2E), which was also found in the natural basement membrane, indicating the biomimetic performance of the scaffold . Nitric oxide is considered to be one of the main vasodilators. It is involved in inhibiting platelet aggregation and is necessary for the functionalization of endothelial cells. The NO measured in the experiment shows that when cultured on a biomimetic double-layer scaffold, the formed endothelial cells are functional and have the ability to send signals to vsm-like cells.
In addition, large pores and thicker near-field electrical direct-write fibers can induce rapid infiltration of vsm-like cells, thereby satisfying MSC/ECFC co-culture (Figure 2J) and MSC monoculture. The inoculated MSCs covered the entire outer surface of the scaffold (Figure 2G) and arranged in a straight line (Figure 2H). Type IV collagen is also synthesized (Figure 2I), which wraps individual vSMCs into a basement membrane-like matrix.
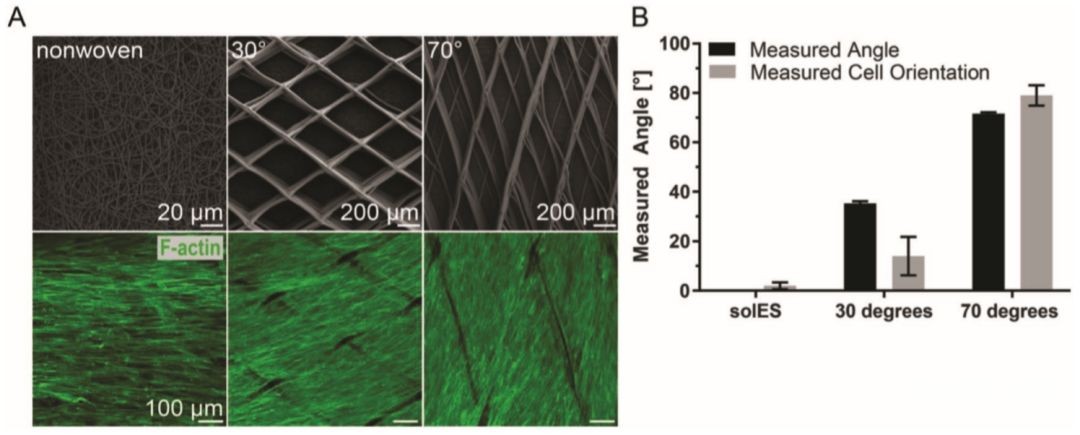
For the outer layer structure of the stent written by near-field electrical direct writing, with the increase of the stent fiber winding angle, the direction of the cells changes to the near-circumferential direction (Figure 3A), which is the same as the original membrane medium. By controlling the orientation of the fibers, the orientation of vSMCs on the tubular scaffold can be guided in a manner that mimics the tissue. The porous structure of the outer layer promotes the rapid growth of cells and produces several layers of oriented cells with close interactions between the cells.
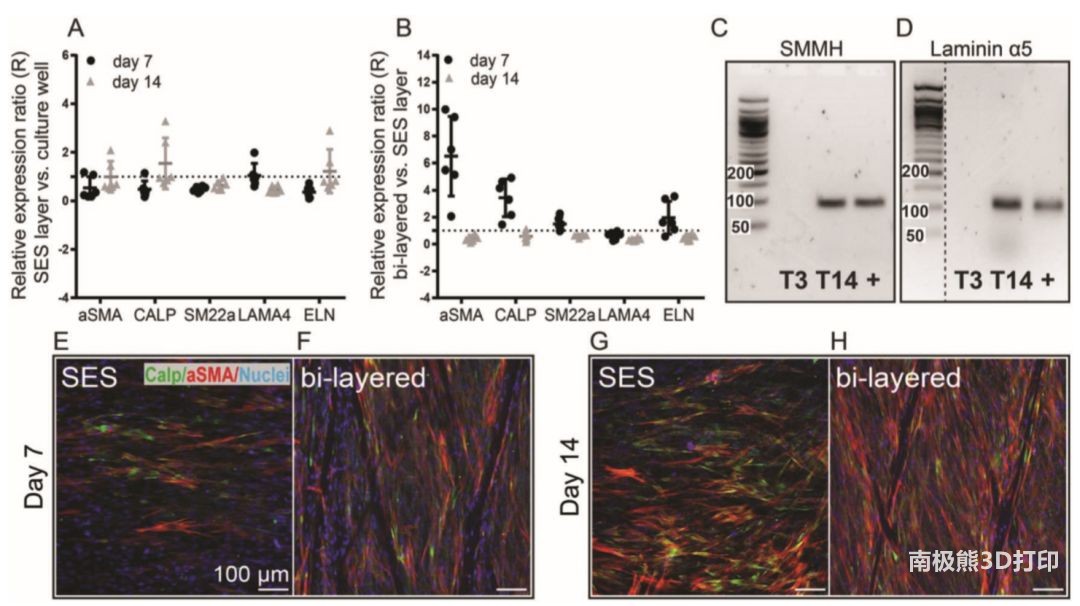
In order to further investigate the influence of the interaction of cells on the large-pore size scaffold on the differentiation of MSCs. The researchers compared the results of cells with and without outer fused electric direct write fibers. The results showed that the MSCs cultured on the orifice plate and the monolayer solution spinning scaffold had similar relative gene expression levels. The gene expression levels of MSCs cultured in a double-layer vascular structure and MSCs cultured in a single solution spinning layer are quite different. The experimental results of DNA and protein expression are also similar.
This heterogeneous scaffold with a composite structure has a structure similar to the inner and outer membranes of the native blood vessel. The inner solution spinning layer has a small diameter and low cell permeability, which can promote the formation of an endothelial cell layer; and a low fiber density, controllable deposition orientation The porous outer membrane is the basis for realizing the directed and rapid colonization of vsm-like cells. It does not require soluble factors and surface functionalization, and the structural design close to release guides and guides the morphology and differentiation of cells. In the future, we can also explore whether we can use this heterogeneous design to adjust the immune response and make it develop in the direction of regeneration.
Paper link:
https://doi.org/10.1002/adfm.201905987
(Editor in charge: admin)


0 Comments for “Electrospinning + Near Field Direct Writing Printing Small Diameter Blood Vessel Stent”