Introduction: March 11, 2022, German chemical company
Evonik
(
Evonik
group)
Invest
span3D printingPharmaceutical company Laxxon Medical.Through the fusion of two unique patented technologies, Laxxon provides (SPID) core technology and Evonik provides novel tablet targeted delivery, intended to promote3D printingLarge-scale commercial mass production of tablets.
Evonik
medical
Thomas Riermeier, Head of the Healthcare Business Line: “By3D printing technologyIt can improve the precision of drug delivery, and can benefit specific patient groups in a more targeted manner. ” “The cooperation with LAXXON will jointly accelerate our development in the medical market. “

3D printing pharmaceutical technology has been evolving
Compared with traditional pharmaceuticals, 3D printing
clinical
Drug development has certain competitive advantages, such as providing more precise personalized and targeted drug delivery services for individual patients. At the same time, 3D printing drugs have made considerable progress, but the technology still needs more clinical cases and manufacturing technologies to be breakthroughs before it can truly achieve commercial mass production.
Aprecia’s 3D printed Spritam epilepsy drug is currently the only FDA-approved drug, although other companies are not far behind.For example, global pharmaceutical company Merck and
EOS
The group company AMCM has embarked on a joint project to develop and produce 3D printed tablets, which are planned for clinical trials and then for commercial mass production.
Meanwhile, researchers at the University of London, the University of Southern California and FabRX have succeeded in making drug-loaded tablets in seconds using volumetric 3D printing, a faster method of 3D printing pharmaceuticals.

Evonik and Laxxon join forces to commercialize 3D printed pharmaceuticals
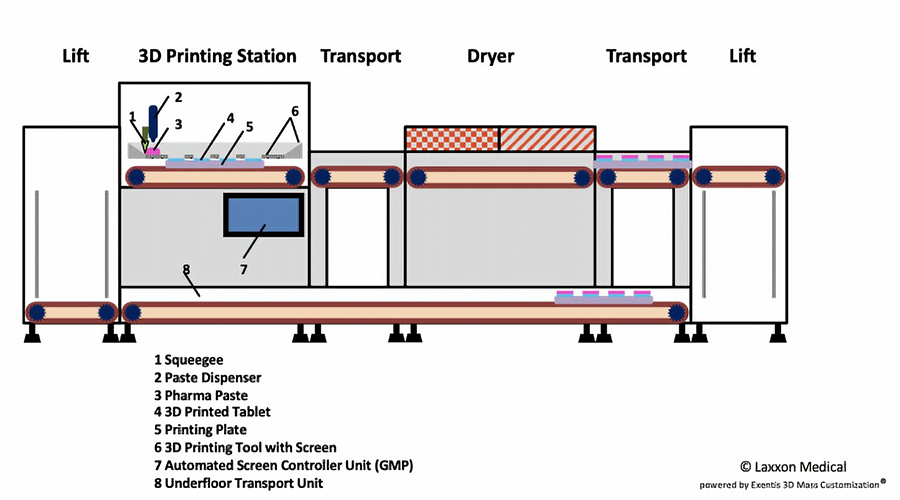
Laxxon Medical is a US-based company that has developed a patented 3D screen printing technology capable of controlling the release of multiple active pharmaceutical ingredients in a single tablet.
Its 3D screen printing technology enables the internal structure of the pill to contain alternating layers of active and inert layers that release different doses of the drug over time. In other words, the company’s screen-printed innovative drugs (SPID) technology allows multiple drug ingredients to be combined in a single pill through a 3D-printed layer, enabling several pills to be combined into one.
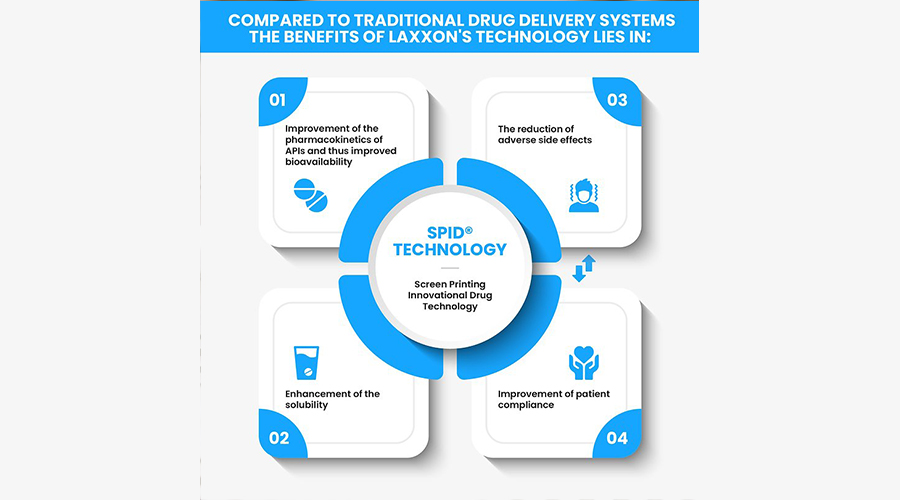
Another advantage of Laxxon’s screen printing technology is its manufacturing speed, with the company claiming that SPID technology is “much faster” than all 3D printing processes at this stage, enabling mass commercial production.
“This technology is very useful for patients,” said Bernhard Mohr, head of venture capital at Evonik. “We expect that more controlled delivery of the drug will result in fewer side effects, and taking fewer pills may reduce the likelihood of forgetting to take a pill during the day.”
With 60 years of experience in delivering drug systems, Evonik manufactures excipients which are inactive ingredients that act as drug carriers, for example as tablet coatings and allow drug release over a sustained period of time.
“Evonik is the perfect partner to support the development of tablets with unique release properties,” said Helmut Kerschbaumer, co-founder of Laxxon Medical. “We are delighted to be able to collaborate with the world’s leading specialty chemicals company, through which we can further develop our products and simultaneously commercialize them in mass production.” Evonik’s proprietary polymers ensure the targeting of active ingredients in novel tablets delivery.
(responsible editor: admin)


0 Comments for “Evonik invests in Laxxon for technical cooperation to promote commercial mass production of 3D printed medicines”