It is understood that the FDA document is issued in the form of a discussion document, which not only outlines the point of care3D printingThe management approach also identifies end-use challenges and provides revised potential specifications. The FDA stated that these recommendations are not intended as guidance, but are intended to “raise questions,” so it is now requesting medical treatment.3D printing industryThe feedback provided by the company provides information for future supervision.

According to William Maisel and Ed Margerrison of the FDA OSEL and CDRH divisions, 3D printed medical devices are at the forefront of innovation and healthcare. The FDA used discussion papers to gain insight into the benefits and challenges of 3D printing in hospitals and other points of care, and proposed a potential regulatory approach. The document aims to promote discussion and solicit public feedback, and lay the foundation for the development of appropriate 3D printing supervision methods in point of care, patient personalized care and field innovation.
According to the FDA, in hospitals and
Operation
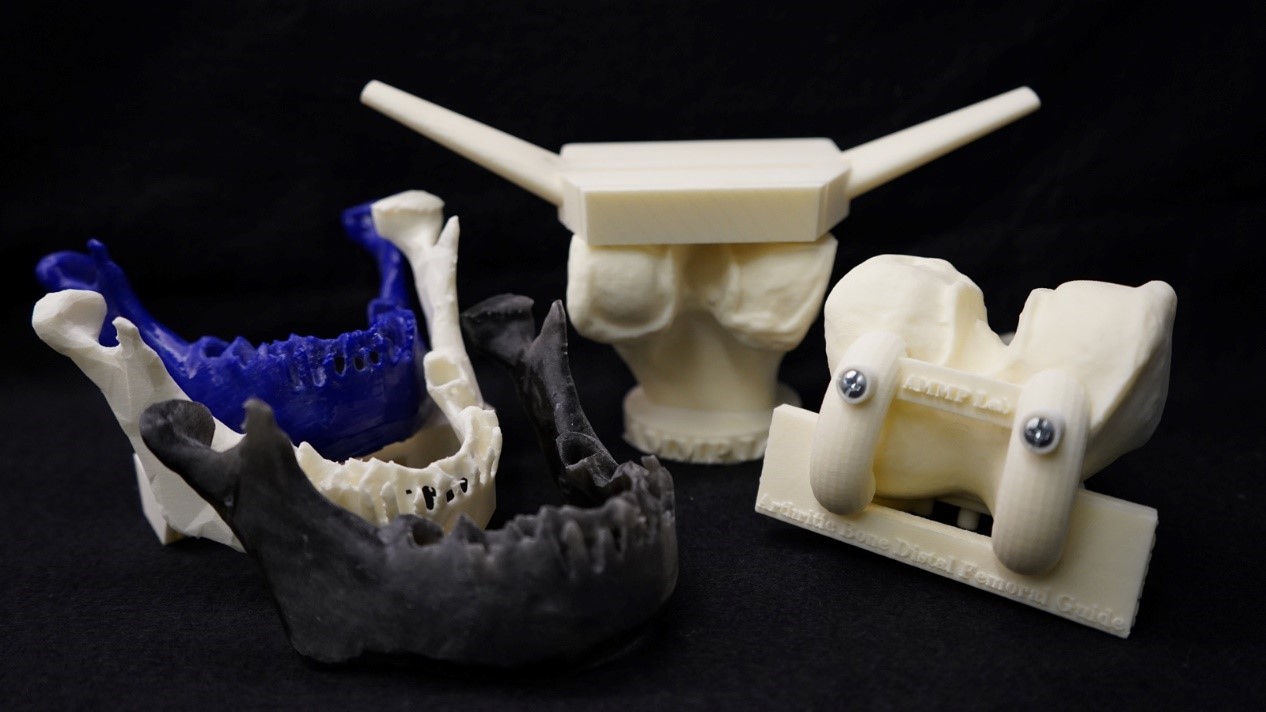
The use of 3D printing in China can quickly produce personalized equipment for patients, such as anatomical models and other instant medical devices. The article also emphasized the role of the technology in helping to solve supply chain issues, such as the global equipment shortage that occurred in the early stages of the new crown epidemic.

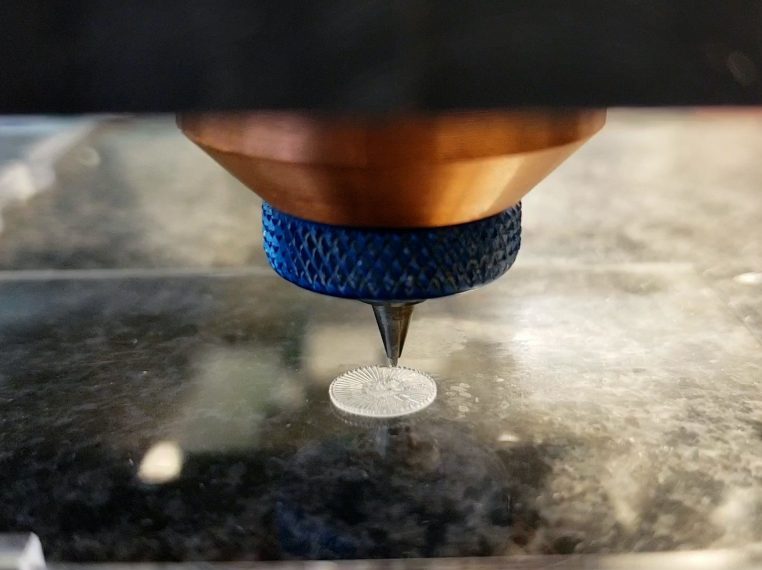

- On-site installation by healthcare institutions3D printer
- Hospitals or operating rooms also use related procedures
- Contract with a nearby service bureau to produce and deliver medical equipment
1. Discussion Paper: 3D Printing Medical Devices at thePoint of Care
2. 3D Printing Medical Devices at the Point of Care: Discussion Paper
3. FDA ISSUES CALL FOR FEEDBACK ON THE REGULATION OF 3DPRINTED MEDICAL DEVICES
4. ONKOS SURGICAL RECEIVES FDA CLEARANCE FOR NEW 3D PRINTEDBIOGRIP COLLARS
(Editor in charge: admin)


0 Comments for “FDA publicly solicits feedback on 3D printing medical device supervision mechanism”