
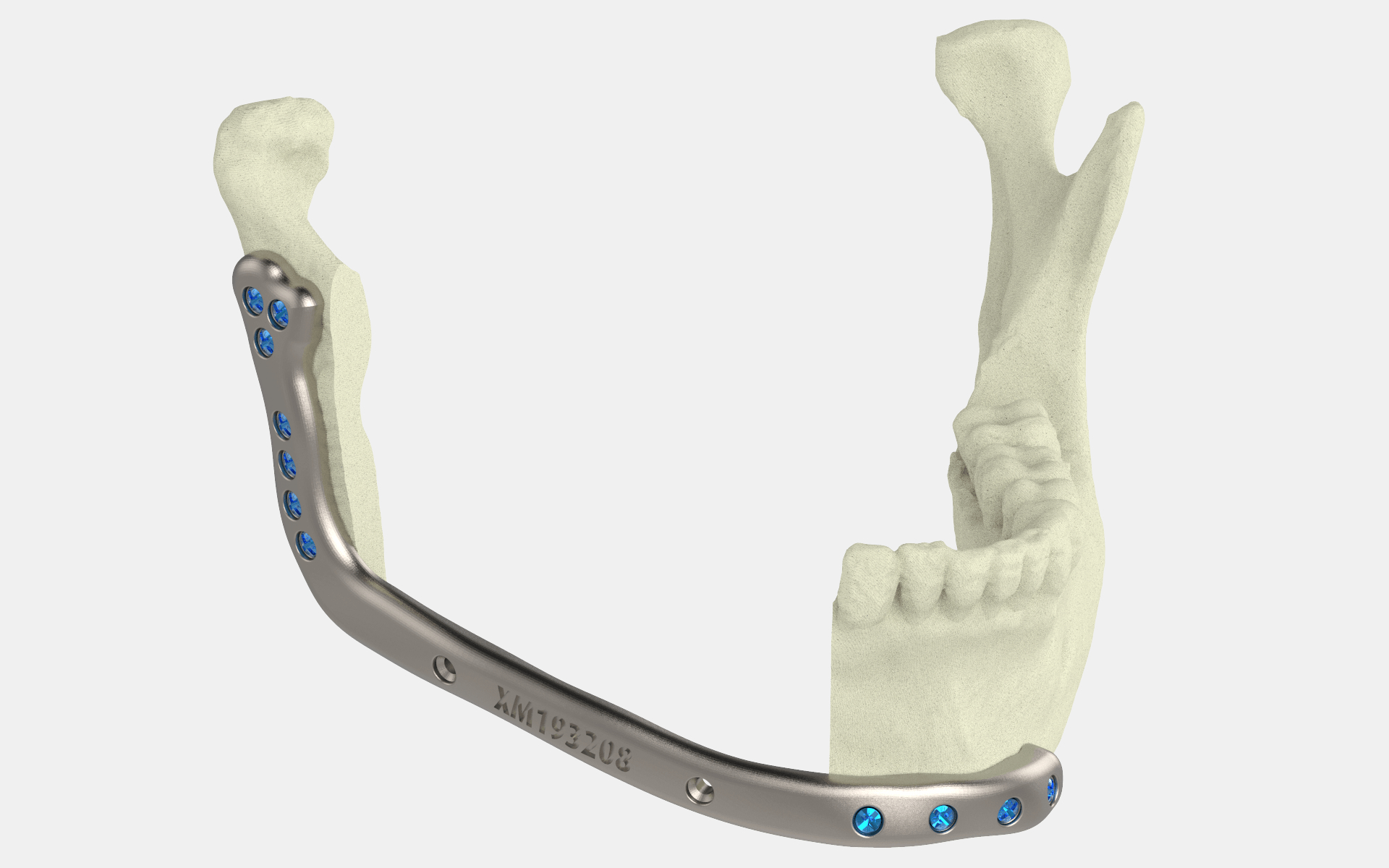
For patients diagnosed with oral cancer, removal of part of the mandible is sometimes a necessary procedure. In these cases, mandibular reconstruction surgery is usually used to normalize the contour of the lower face, regain structural support, and improve the relationship with the teeth. The operation can make patients more powerful in speaking and chewing, and greatly improve their quality of life.
Health Canada approved the use of Specifit 3D mandibular plate in September 2021,
surgical
Doctors can use the implant and two surgical cutting and drilling guides to treat patients.
According to this approval, the 3D Specifit mandibular plate must be used
biology
Compatible materials for 3D printing. Medical experts use grade 23 titanium to produce this implant, using laser powder bed melting (for preparing implants) or electron beam melting (for two surgical cutting and drilling guides).
Since the design of the device is customizable, the patient’s specific treatment needs will greatly benefit. The implant can be 3D printed according to the patient’s anatomy, which will help increase the success rate of surgery and reduce surgery and recovery time.
CRIQ Vice President Lyne Dubois added: “The approval of LARA 3D fully reflects CRIQ’s leadership in the field of additive manufacturing in Quebec. The Specifit series of 3D prostheses will meet the needs of the healthcare sector and provide patients with better Quality of life. It also showcases the wide range of possibilities that 3D printing offers for companies that need to design complex, lightweight and durable parts. The professional experience accumulated by CRIQ can be used as a reference or reference for the entire 3D printing ecosystem in Quebec.”

(Editor in charge: admin)


0 Comments for “Health Canada has approved its first domestically manufactured 3D printed medical implant”