Using advanced 3D printing technology, Stanford University researchers have transformed a paste made from living cells into hearts and other organs.
The heart is the most amazing organ of the human body. Its chambers can pump evenly for a long time, and the material is flexible and can be contracted on demand. It’s a structural marvel — however, when something goes wrong with the heart, its inherent complexity makes it a real challenge to repair. As a result, thousands of young people with congenital heart disease have to deal with the disease for life.
Stanford University
biology
“Pediatric heart disease is one of the most common congenital disorders in the United States,” said Assistant Professor of Engineering Mark Skylar-Scott. “It creates enormous difficulties for families. Several approaches can be
Operation
Extending the lives of children who still experience limited mobility and life of uncertainty. A truly healing solution can only be achieved by replacing damaged or misshapen tissue in some way. “

That’s exactly what Skylar-Scott, who is working on new ways to treat congenital heart disease by building engineered heart tissue in the lab. It’s not just growing cells in a dish, Skylar-Scott points out. Most existing technologies implant heart cells or stem cells into a temporary “scaffold”: a porous, spongy substance that holds the cells in place in three dimensions. Although this method allows researchers to grow tissue in the lab, it only works with extremely thin layers of cells.
“If your scaffold is only a few cells thick, you can put the cells in the right place. But if you’re trying to grow something a centimeter thick, it’s hard to grow the cells in the right place so that they can grow tissue, That makes it difficult to keep cells alive.” Human organs are also not single spheres of cells, each made up of complex layers of multiple cell types, resulting in 3D structures that are difficult to replicate, Skylar-Scott adds.
printing organoids
To solve the organ growth problem, the Skylar-Scott team uses advanced 3D printing technology, where they create one thick layer of tissue at a time and place the desired cells in the right places. Skylar-Scott points out that this construction method is ideal for replicating complex tissues such as the heart, where 3D form is important.
Despite its promise, 3D printing of cells also presents some deep and intractable challenges. Unlike consumer 3D printers, which can heat and extrude materials in countless shapes, cells are alive and fragile.
“If you try to place one cell at a time, it could take hundreds or thousands of years to print a liver or heart,” says Skylar-Scott. “Even if you place 1,000 cells per second, you still need to place billions of cells to get an organ.”
Instead, the Skylar-Scott team is speeding up the printing process by placing dense clumps of cells called “organoids.” The team produced clumps by placing genetically modified stem cells in a centrifuge, which resulted in a paste-like substance. Using this mixture, they were able to simultaneously print large numbers of cells into gel-like 3D structures. Large-scale structures of organs are achieved by printing these organoids.
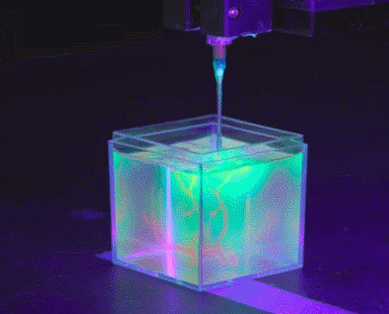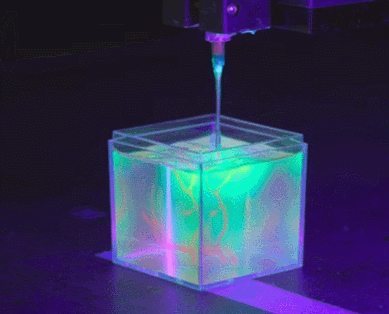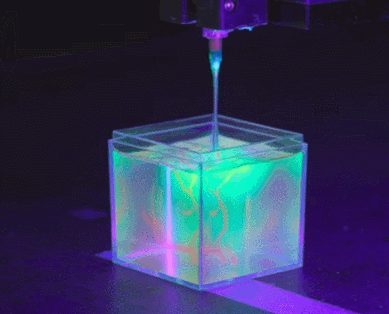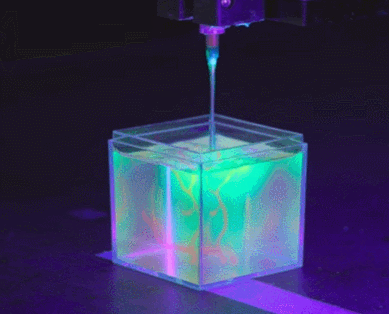
cell programming
However, getting stem cells in the right place is only the first step. Once they are printed, researchers must somehow differentiate them into more specific cell types, forming a multi-layered cluster of working cells that resembles healthy organ tissue. To do this, Skylar-Scott soaked stem cells in a chemical mixture.
Each type of stem cell is genetically engineered to respond to a specific drug, and once they sense the drug, the stem cells differentiate into specific cell types. Some cells are programmed to become cardiomyocytes, the heart cells that form the core functional tissue of the heart. Others are programmed to become stromal cells, which hold the tissue together.
Skylar-Scott tested the printed tissue in a smartphone-sized bioreactor, which helps keep the printed cells alive. The research team grew an organ-like structure in the bioreactor: a tube about 2 inches long and half a centimeter in diameter, like a vein in the human body, a tiny device that pumps, contracts and expands on its own to allow fluids to flow. through itself.

expand
Skylar-Scott quickly realized that a larger structure, such as a functional chamber that could be implanted into an existing heart, should be printed, but it still has a long way to go. This scenario implies a 16-fold increase in the size of the experimental venous pump. To produce something closer to that size, a whole new organ, the lab would need to dramatically scale up cell production.
“Scaling an artificial heart would only require building a bigger printer,” Skylar-Scott said. “The key is still the cells themselves.”
“Right now, it takes a month to grow enough cells to print something tiny, but it’s also very expensive, costing tens of thousands of dollars per test. We need to figure out ways to engineer cells to make them more robust, and the cost of growth Lower. Once the pipeline for new cells is in place, I think we’re going to start seeing some incredible progress,” Skylar-Scott said.
Original link:
https://news.stanford.edu/2022/0…art-one-layer-time/
© THE END
(responsible editor: admin)


0 Comments for “How is Stanford speeding up 3D printing hearts?”