China3D printingMedical device maker 3D LifePrints has announced plans to bring its point-of-care medical equipment to life by opening a new facility in Texas.3D printingService expands to the United States.
Notably, the move marks the company’s first international expansion, followed by several new point-of-care centers in other U.S. states.
The company is also supporting its U.S. team by including physician and former NASA astronaut Dr. Scott E. Parazynski. Parazynski will join the company’s leadership team and board of directors to assist with its long-term strategic initiatives across the state.
“3D printing is revolutionizing the global healthcare industry, providing surgeons and clinicians with patient-specific products and services to improve patient outcomes and reduce costs,” said Paul Fotheringham, founder and CTO of 3D LifePrints. “We are excited to begin our international expansion in the United States, where we have been able to offer a range of state-of-the-art 3D medical solutions.
“We are also honored to welcome Dr. PARAZYNSKI to our leadership team; attracting such a fine figure is a testament to the ambition of our company.”

3D LifePrints is expanding its bedside medical 3D printing center models to the United States. Photo via 3D LifePrints.
From humanitarian aid to international expansion
3D LifePrints started out as a humanitarian aid organization providing 3D printed prosthetics to amputees in Africa. Since then, the company has expanded to build four “innovation centres” in the UK from which to 3D print surgical models to help patients better understand their conditions and provide surgeons with tools for preoperative training.
The centers are equipped with FDM, SLS, SLA and Polyjet 3D printing technologies and have grown in size over the past few years due to successive funding rounds. In 2018, 3D LifePrints secured an investment worth £500,000 to continue developing its 3D printing facilities embedded in various UK hospitals.
In March last year, the company raised a further £1.2 million to bring its hub-based model to more cities in the UK, touting international expansion plans to come. That vision was backed up in June this year when the quality assurance process behind its 3D printed medical models and devices received ISO certification.
Now, the company’s international ambitions have become a reality as it hopes to open the first medical 3D printing factory in the United States.
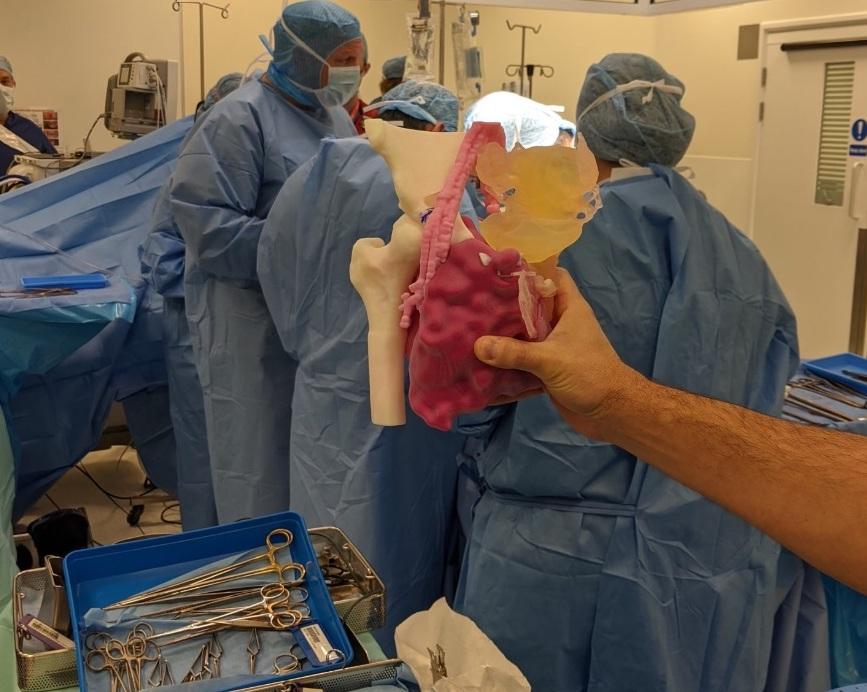
A surgeon holds a 3D LifePrints 3D printed heart model.
3D LifePrints’ embedded QMS is ISO 13485:2016 certified
National Medical3D printingServe
3D LifePrints is supported by the British Association of Health Technology Industries (ABHI), which is currently part of a US accelerator program in partnership with the Dell School of Medicine in Austin, Texas.
The company will build out its center-based models in the U.S. by opening embedded medical 3D printing facilities across Texas. The centers will partner with leading U.S. hospitals to provide instant in-house medical 3D printing services, helping hospitals reduce operating costs and improve patient outcomes.
The 3D LifePrints team will work closely with surgeons and clinicians at the host hospital to provide virtual and 3D printed patient-specific anatomical models, as well as custom-made surgical stimulation and training equipment. These medical devices will be used in a wide range of surgical specialties such as pediatrics, cardiothoracic, orthopedics, oncology and craniomaxillofacial.
With 3D LifePrints’ point-of-care services, surgeons and clinicians can leverage the expertise of on-site biomedical engineers and take advantage of a range of 3D printing hardware and software products. Embedded hub models will enable hosting hospitals to benefit from in-house 3D printing services, with engineers having easy access to and immediate delivery of equipment. The company will also provide remote services through its digital platform EmbedMed.
The opening of the new center will mark the company’s first international expansion, with more point-of-care centers to follow in several other states, but potential locations have not been announced.“We are delighted to hear that 3D LifePrints has been successful in the United States and its unique business model and technology are helping advance care for patients and clinicians,” said Paul Benton, Managing Director, International ABHI. “I am delighted that through programmes like the US Accelerator, UK companies can find opportunities to connect with new partners and expand the advantages of their services.”
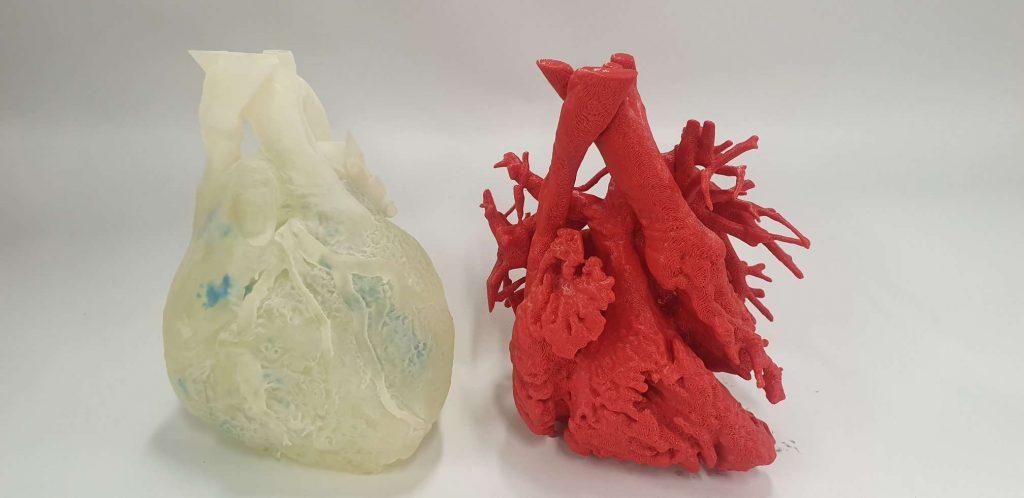
3D Lifeprints’ technology has been used in a range of medical applications, including soft-printed models for simulating manipulations (pictured). Photo via 3D Lifeprints.
China3D printingNet compiled articles!
(responsible editor: admin)


0 Comments for “Medical device maker 3D LifePrints expands point-of-care 3D printing service to US”