China3D printingNet March 10th, drug testing and screening for cancer drug discovery may take years, and the 2D cell cultures and animal models used to evaluate its efficacy are usually not representative of the human body, which is why researchers turn to biology The reason for printing technology to improve the success rate. Improve efficiency in human trials by providing human-specific preclinical data. In 2018, there were 17 million new cancer cases worldwide, and the disease is expected to affect 27.5 million people every year by 2040. This is why researchers are considering adopting new technologies that can accelerate drug discovery. Although still in the laboratory stage, new technologies using immersive biological humanoid biosignatures may change the drug screening mechanism.
Researchers from Cornell University, Wake Forest School of Medicine, Virginia Tech and State University, and Ohio State University published an article demonstrating an immersion printing technology that can perform tissue and organoids in a 96-well plate. Bioprinting can improve the throughput screening of 3D drugs. Using a hydrogel bio-ink composed of hyaluronic acid (HA) and collagen, they were able to bioprint it into a viscous gelatin bath, thereby preventing the bio-ink from interacting with the pore wall and providing support to keep it spherical.
According to China3D printingThe network understands that the use of bioengineered organoids based on human cells can not only increase the likelihood of success in human trials, but can also be used for personalized medical diagnosis to optimize the treatment of diseases such as cancer. However, they believe that the limitation of using organoids in drug screening is that it is difficult to create a large number of homogeneous organoids with form factors compatible with high-throughput screening, so bioprinting can be used to expand such organoids and tissue constructs Of deposition.
The team of scientists used two commercially available bioprinters to evaluate the compatibility of collagen HA hydrogels and HyStem-HP hydrogels: Cellink’s INKREDIBLE bioprinter and Allevi’s Allevi2 bioprinter. This method has been validated using a variety of cancer cell lines, and then applied to patient-derived glioblastoma (GBM) (a fast-growing brain tumor) and sarcoma (or malignant tumor) biological specimens for drug screening.
To initially analyze the biocompatibility of the hydrogel, the researchers used two common cell lines: human liver cancer and human colorectal cancer.
When processing patient-derived tumor biological specimens, they followed the guidelines of the Wake Forest Baptist Medical Center IRB protocol and obtained two glioblastoma and one sarcoma biological specimens from three patients undergoing surgical treatment. These biological samples were processed into cell suspensions, and millions of living cells were successfully generated from each sample. The cells are then combined with collagen-HA bio-ink for immersion bioprinting. After bioprinting, the tumor organoids (PTO) derived from GBM and sarcoma patients were kept in the incubator for 7 days, and then the chemical screening study was started.
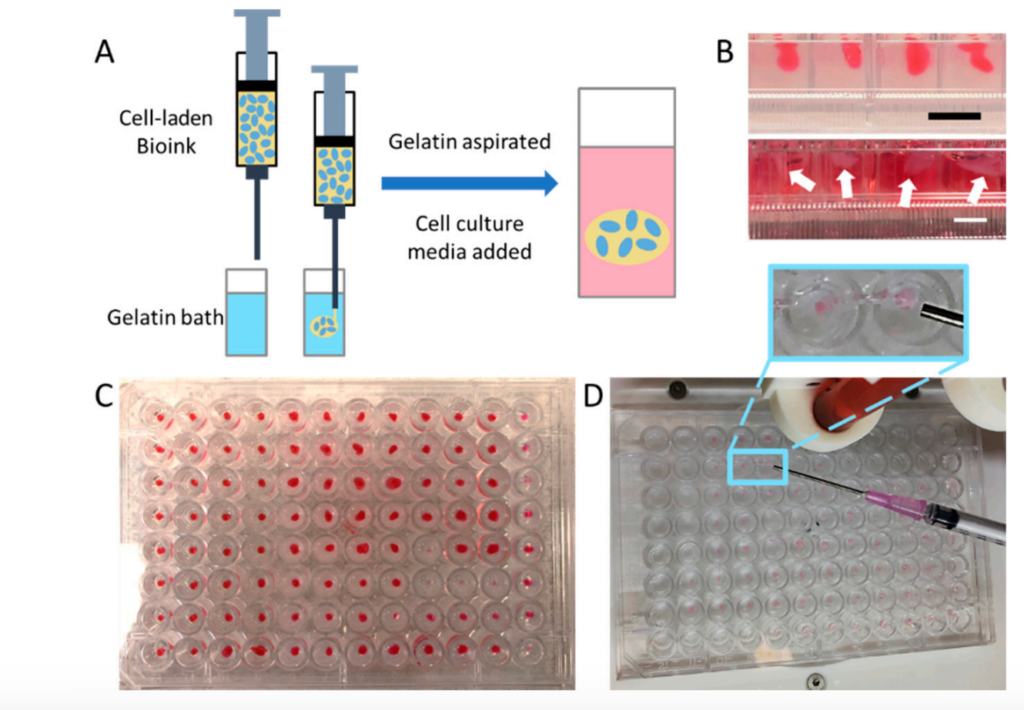
Schematic diagram of the printing process using two types of bio-inks in two commercially available bio-printers: Cellink Inkredible and Allevi 2 (Photo: Cornell University/Wake Forest)
The researchers claim that although their PTO is useful for disease modeling, mechanism research, and drug development, they also use these models to influence treatment from a diagnostic perspective, which may only be the ultimate goal of their work.This is called immersion bioprinting3D bioprintingThe method is an effective method that can overcome the limitations that plague the tumor organoid system. In this case, experts suggest that the printing process itself is a method or new and more user-friendly bio-ink production capacity. “
China3D printingOnline reviews:From ineffective trial design to integrating new technologies into diagnosis and drug tracking, clinical oncology has faced severe challenges in the past decade. However, advances in new methods ranging from hardware design to improved bio-inks developed specifically for bioprinting have opened up new opportunities for bioprinting-based applications. This new research especially shows that with the development of bioprinting hardware, software, functional ECM-derived bio-inks and the modification of the printing protocol, bioprinting can not only be used to print larger tissue structures, but also can be used for a large number of Micro-organisms and tumor models for drug development, diagnosis and personalized medicine applications.
China3D printingNet original article!
(Editor in charge: admin)


0 Comments for “New method: 3D printing of immersed tumor organisms will increase drug screening throughput”