China3D printingNet July 5th, Orthofix Medical, a Texas-based developer of 3D printed orthopedic equipment, has launched its new FORZA Ti PLIF spacer system.
Titanium implants are designed for posterior lumbar interbody fusion (PLIF) surgery, with complex 3D printing geometry, porous structure and nano-scale surface texture, which can promote bone growth through spacers. In addition to the product launch, Orthofix Medical also announced the first patient implant to use a lumbar intervertebral device, and there will be more in the future.
“The FORZA Ti PLIF spacer system using Orthofix’s unique Nanovate technology is one of several new 3D printed titanium products we have recently launched,” said Kevin Kenny, Orthofix’s global spine president. “The FORZA Ti system is also very suitable for use with our flagship Trinity ELITE allograft, which contains living cells and provides the necessary ingredients for new bone formation.”
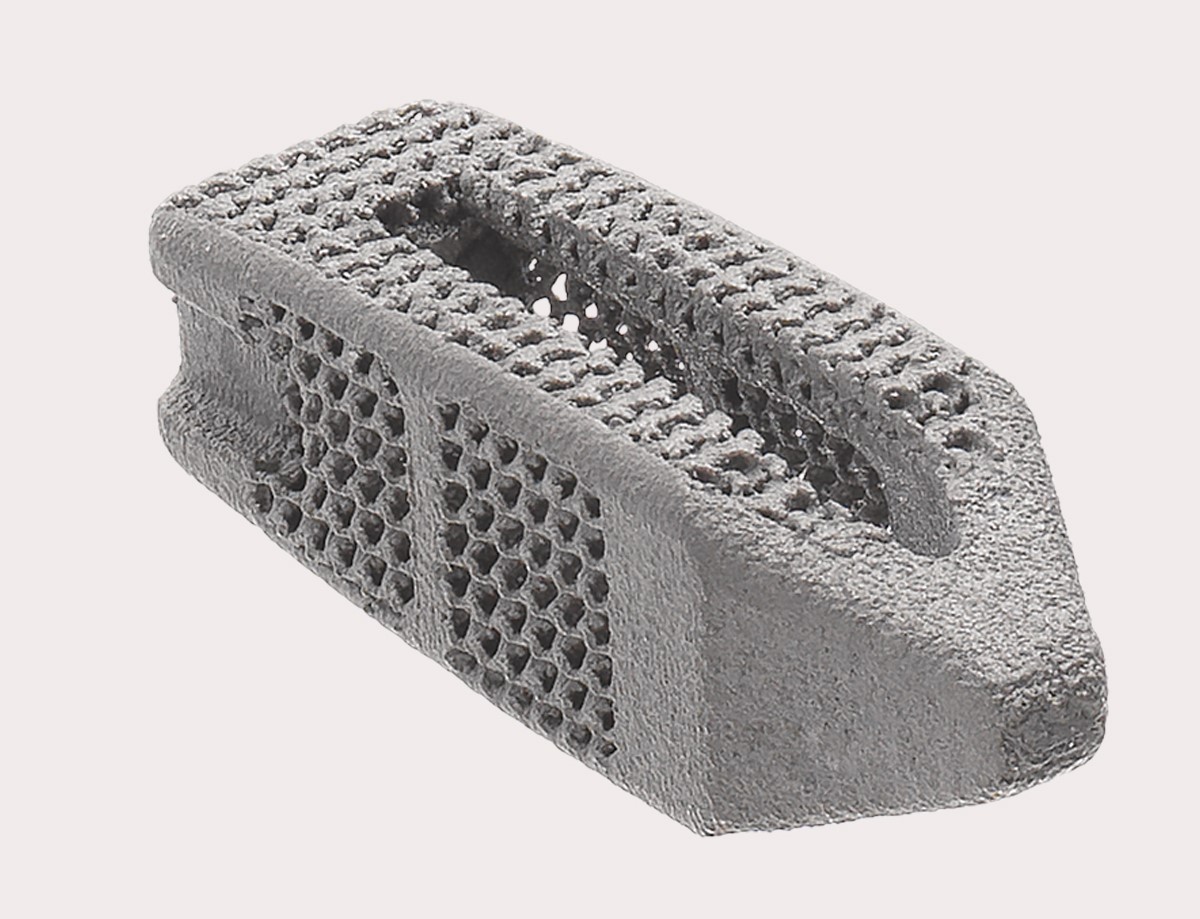
3D printed FORZA Ti PLIF gasket system. Photo from Orthofix Medical.
Posterior lumbar interbody fusion
PLIF is a form of lower back spinal fusion surgery in which a bone graft or synthetic cage is added to the spinal area to initiate a biological response that promotes bone growth between two vertebrae.
Unlike some other forms of spinal fusion, PLIF usually requires the insertion of an intervertebral device between the vertebral components, which helps to reduce the pressure on the local nerves while holding the vertebrae in place for fusion. The FORZA Ti PLIF gasket system is a typical example of this type of equipment.
According to Dr. Joel Siegal, a neurosurgeon at St. Vincent Charity Medical Center who performed the first FORZA implant operation, “The ability to maximize bone ingrowth is essential to the success of the fusion process. The large amount of materials used to fill bone grafts The opening and the 3D printed titanium endplate of the FORZA Ti Spacer have been carefully designed to help us achieve the goal of maximizing bone ingrowth to aid fusion.”
FORZA Ti PLIF Gasket System
At the top of the large open graft window, the FORZA spacer system has many built-in features designed to increase the success rate of the PLIF procedure. This includes a nose with a bullet that can disperse the spine more easily. The titanium structure of the implant also has a nano-scale surface texture, which has been proven to improve bone proliferation and alkaline phosphatase activity in human stem cells (this is an early bone cell differentiation marker).
In addition, the porosity of the implant midline is 80%, which can increase the perspective visualization. At the same time, the 50% porosity endplate has an interconnected gyro structure and 400 micron holes, which helps promote better bone ingrowth.
In addition to the FORZA Ti PLIF gasket system, Orthofix has recently launched many other 3D printed medical equipment, such as CONSTRUX Mini Ti gasket system, CONSTRUX Mini PTC gasket system and Pillar SA PTC gasket system.
The porous titanium structure of the implant is designed to promote bone ingrowth. Picture from Orthofix Medical.
Additive manufacturing has found its place in the medical field, and end-use implants are now 3D printed. Onkos Surgical is a supplier of orthopedic oncology medical equipment. Its modular 3D printed BioGrip collar product portfolio has recently received a 510(k) clearance from the U.S. Food and Drug Administration (FDA). Much like the FORZA Spacer, the porous medical device is designed to support bone growth in bone cancer survivors and other complex limb rescue cases.
Elsewhere, in the field of materials, metal 3D printing expert Z3DLAB recently revealed that it is currently developing “new generation materials” with enhanced biocompatibility and potential 3D printed implant applications. The company will work with the French CNRS Scholars Alliance to develop a “composite enriched alloy” with the characteristics required for SLM 3D printed surgical implants.
China3D printingNet compile article!
(Editor in charge: admin)


0 Comments for “ORTHOFIX MEDICAL launches a new 3D printed lumbar spine device for spinal fusion surgery”