China3D printingNet, June 29, a 3D printed bionic graft developed by a multidisciplinary team at the Wyss Institute at Harvard University led by Gliklich Healthcare Innovation postdoctoral researcher Nicole Black is seeking a 510K license from the U.S. Food and Drug Administration (FDA).
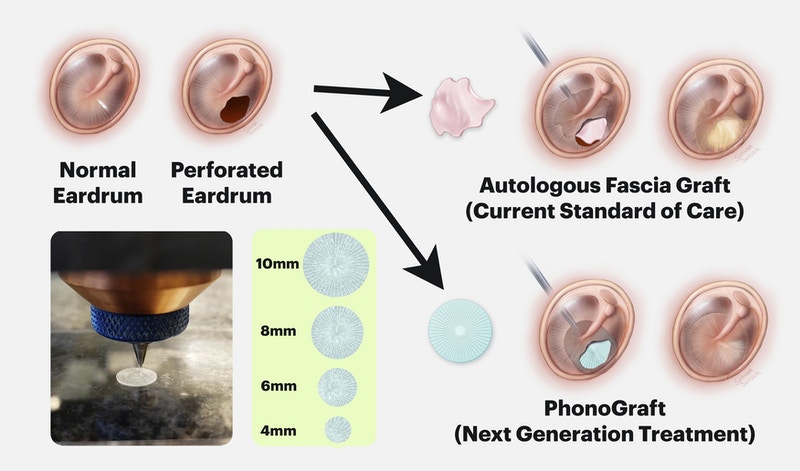
The device, called PhonoGraft, is designed to imitate the tympanic membrane of the human body and can be used for patients with ear trauma, so that the tympanic membrane can repair itself by producing new cells.
It is hoped that 3D printing equipment can increase the speed and success rate of tympanic membrane repair surgery, while reducing the cost of surgery for patients.
How PhonoGraft (blue) causes the function and form of the tympanic membrane to regenerate. Image courtesy of Shawna Snyder / Wyss Institute.
Development3D printingeardrumGraft PhonoGraft
Perforation of the tympanic membrane due to trauma, chronic ear infections, and abnormal skin growth can lead to severe hearing loss and repeated infections. According to the Wyss Institute, this happens to approximately 30 million people worldwide each year.
When these perforations cannot heal on their own, they need to undergo a reconstructive “tympanoplasty” surgery, which uses graft materials taken from other parts of the body (such as fascia) to repair the defect. However, these grafts do not always ensure that the tympanic membrane is completely repaired, which means that patients often need to undergo re-surgery and may suffer long-term hearing loss.
In response to the need for better and longer-lasting tympanic membrane reconstruction methods, Black began developing PhonoGraft six years ago, in collaboration with Dr. Aaron Remenschneider of Massachusetts Eye and Ear Hospital in Boston and Professor Jennifer Lewis of Harvard University.
PhonoGraft is a biomimetic graft 3D printed from a biodegradable elastomer. Its function is similar to that of a natural eardrum, and thanks to the use of 3D printing, it can adapt to different ear sizes and requirements. Once inserted into the patient’s ear, voice transplantation can effectively transmit sound and promote the growth of natural tissues and blood vessel cells, while slowing biodegradation. As a result, new tympanic membrane tissue is regenerated in the ear, can conduct sound and provide a normal barrier function against pathogens.
“PhonoGraft promotes tympanic membrane regeneration by combining biodegradability with its biomimetic structure composed of circular and radial features,” Black said. “This kind of graft can conduct sound well at both low and high frequencies, so it is much better than the autologous tissue grafts currently used to restore function.
In addition, we hopePhonoGraftIt will improve the patient’s healing and hearing outcomes, thereby reducing the need for revision surgery. “
The road to FDA approval
Black and her team have previously verified Phonograft in pre-clinical in vivo studies, using a Chinchilla model with eardrum size and hearing range similar to humans. They observed that PhonoGraft promoted the remodeling of the natural tissues of chronically perforated animals while slowly degrading, and conducted auditory tests to determine that the 3D printing device was “more effective” in restoring hearing than traditionally used autologous tissue grafts.
Now, Black is seeking 510K FDA clearance for PhonoGraft, which may enable doctors to “perform dozens of eardrum repairs throughout the day.”According to China3D printingWang understands that she is also committed to designing a voice graft that can be placed in the patient’s ear canal when the patient is awake, requiring only local anesthesia instead of the more time-consuming and expensive general anesthesia.
Looking ahead, once approved by the FDA, Black hopes that PhonoGraft will be marketed to ENT surgeons at the end of next year or early 2023.
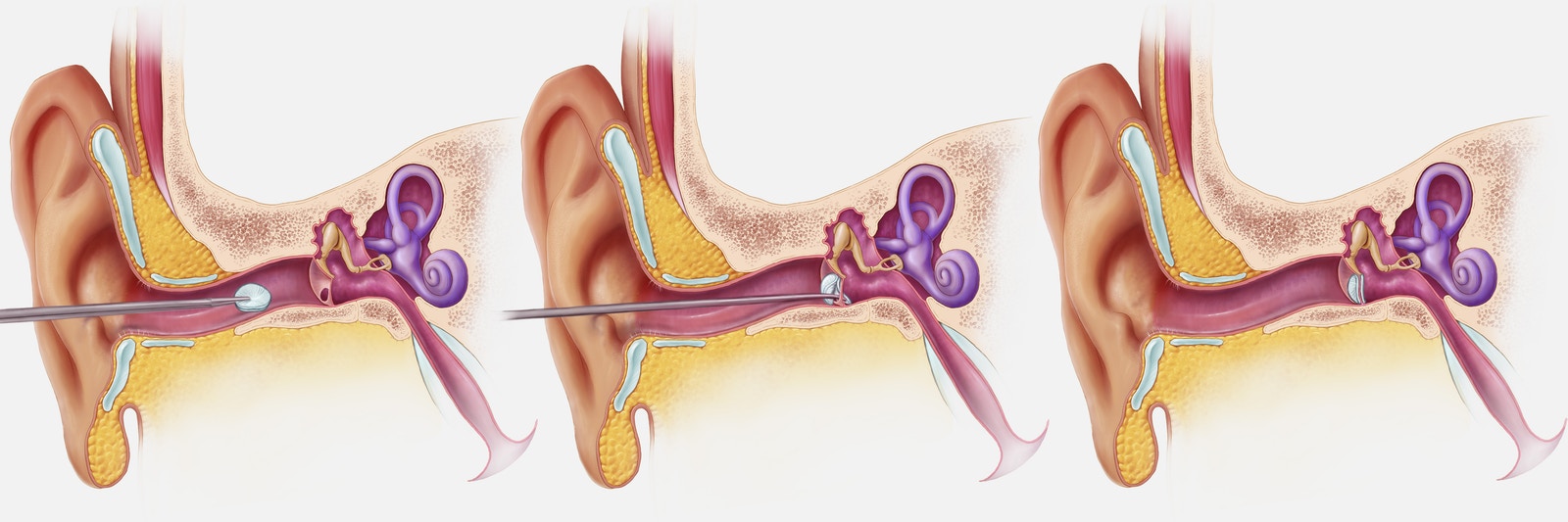
Non-invasive process of installing PhonoGraft through the ear canal. Image courtesy of Shawna Snyder / Wyss Institute.
China3D printingNet compile article!
(Editor in charge: admin)


0 Comments for “PhonoGraft, a 3D printed implant that helps the eardrum heal itself, is applying for FDA approval”