China3D printingNet May 8 news, Bay Area bioprinting company Prellis Biologics is studying the use of biological3D printingLymph nodes to produce SARS-CoV-2 antibodies. Now the idea of using antibodies to prevent diseases is becoming more and more popular,antibodyIt is a protective protein produced by the body due to the stimulation of antigens. It is an artificially produced antibody against the disease by injecting the gel into the patient during the outbreak of an epidemic. To protect humans from infection.
Prellis is working hard to purchase a heat-inactivated virus. The goal is to obtain samples within 14 days of the start of the project. By then, the company will spend about four weeks to print and inoculate human lymph nodes for antibody screening and sequencing. After that, the company suggested that before the partner company mass-produces the antibody, it will be able to find a research center to study the virus neutralization and binding affinity of the antibody.
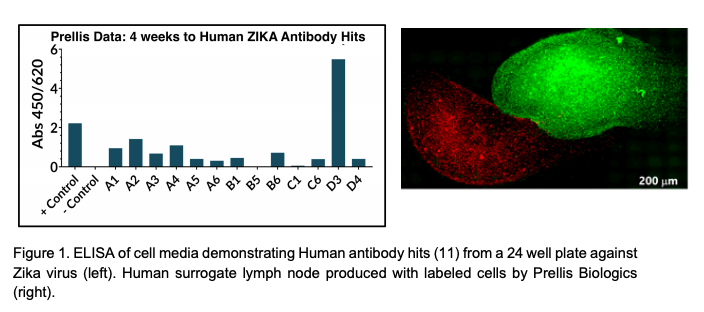
Image courtesy of Prellis Biologics.
Prellis is not alone in producing synthetic antibodies to the SARS-CoV-2 virus. In particular, Dr. Jacob Glanville of Distributed Bio has received widespread attention for promoting this idea. The produced antibodies will be injected into front-line workers, who theoretically will be able to use these antibodies to fight the virus in a short period of time (perhaps eight to ten weeks).
Since 1986, synthetic antibodies have been approved by the U.S. Food and Drug Administration (FDA), commercial companies have studied 570 therapeutic synthetic antibodies in clinical trials, and 79 have been approved by the FDA to enter the market. About 30 of them are used for cancer, and the rest include asthma, arthritis, psoriasis, Crohn’s disease, transplant rejection, migraine and infectious diseases. However, synthetic antibodies for the treatment of viral diseases are still in the exploratory stage.
The first clinical trials of antibody therapy against COVID-19 are currently using an antibody called gimsilumab. The antibody can inhibit the growth of proteins that appear in the serum of COVID-19 patients at high concentrations and is believed to help prevent the lungs fever.
Although we are not experts in antibody production, we certainly should be skeptical of the Prellis COVID-19 project because the risks are so high. From our understanding of bioprinting, it has come a long way since the early 2000s, including many recent achievements in the creation of bioprinted organoids, from the heart to the kidneys to tumors.
However, the application of this technology has not yet been fully realized, and even in drug testing, it is still one of the most direct and feasible uses of bioprinted tissue in the exploratory stage.Some animal studies are currently underway and have broad prospects, for example,3D printingKnee cartilage was successfully transplanted into sheep. That being said, even if Prellis can print out the lymph nodes and see that they produce antibodies, there are many other variables and obstacles to consider when considering the possibility of successful mass production and then using these antibodies as a form of treatment.
According to China3D printingNet understands that the founder, Melanie Matheau, was able to create fully functional human lymph nodes, which produced 11 active antibodies against Zika virus in 2017, and obtained a US patent for this technology in December 2019. Each sample produces antibodies. The company claims that it can perform the same process to develop antibodies against at least one strain of coronavirus currently associated with the global pandemic.
Because the FDA is currently implementing the fast track for emergency approval, many actions have been granted emergency authorization. This includes products that may be problematic, such as the clinical trials of DNA and RNA vaccines conducted by companies working with the US Defense Advanced Research Projects Agency. These companies have not previously been able to obtain licenses for their products for human use because their vaccines cannot be used in humans. Provide sufficient immunity to trial. If Prellis can achieve its goals, it may obtain emergency authorization to perform the Clinical I trial.
China3D printingNet compiled from 3dprint.com
(Editor in charge: admin)


0 Comments for “Prellis Biologics uses biological 3D printing of lymph nodes to produce antibodies to the new coronavirus”