China3D printingNet, September 2nd, the global engineering company Renishaw (Renishaw) and pharmaceutical expert Herantis Pharma have completed the expansion of its phase 1-2 clinical study, the study investigated its nerve infusion drug delivery device and brain The safety and performance of dopamine neurotrophic factor (CDNF) as a treatment for Parkinson’s disease.
according toChina3D printingnetUnderstand that its award-winning nerve infusion drug delivery device is currently the only platform that can promote repeated and intermittent infusion of parenchymal organs (a functional tissue of an organ), which was proven in the first human clinical trial of CDNF.
The preliminary results of this main study are perfect, and the extended part of the study gives all participants the opportunity to receive CDNF infusion to further evaluate the safety, performance and tolerability of nerve infusion and CDNF.
General Manager of Renishaw Neural SolutionsRupert Jones said: “The extended study has been completed, and now I would like to thank the trial participants again for making this research possible and for making personal sacrifices that will ultimately benefit fellow citizens and future Parkinson’s disease patients.”
Parkinson’s disease is a neurodegenerative disease caused by the breakdown of dopamine-producing neurons in the brain. It has debilitating symptoms, including involuntary shaking, muscle stiffness, and slowed movement. Patients also suffer from non-motor symptoms, such as sleep difficulties, memory loss, anxiety and depression. Although symptoms can be treated with medications at first, there is currently no treatment that can prevent disease progression.
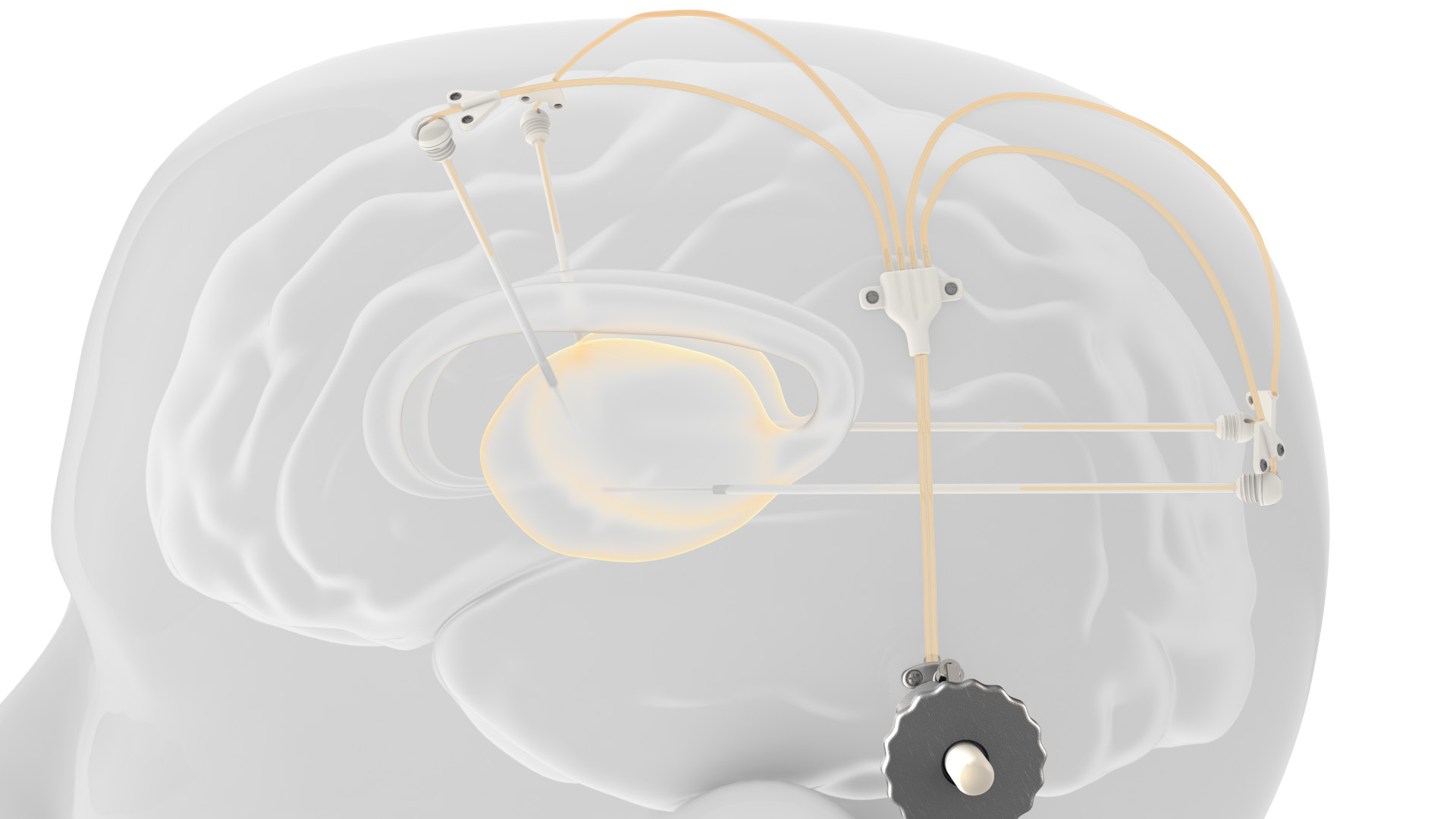
Renishaw’s drug delivery device. Picture from Renishaw.
Nerve infusion intermittent drug delivery system
Renishaw’s nerve injection device consists of four catheters that can be inserted into the patient’s ear3D printingTitanium alloy transdermal port for access, the catheter can be implanted into the target area of the brain.The port is in Renishaw’s own metal3D printingManufactured on the system. The infusion line is connected using an MRI-compatible application kit and placed on the port repeatedly. The retracted needle passes through the septum in the port to allow the therapeutic agent in the external infusion line to be delivered through the implanted catheter.
This patented design means that patients can receive infusions in an outpatient setting without having to implant a new catheter for each infusion, which is the only option so far.
Jones“I’m very pleased to see that Renishaw’s drug delivery system can continue to promote repeated infusions of complex diseases such as Parkinson’s disease for a long time. The performance of this device proves that it is useful for treating many currently incurable diseases. Has a powerful delivery platform for neurological diseases, opening up new possibilities in the fields of neurosurgery and neuroscience.
I think this is a very positive step forward, and I believe everyone involved in the research should be proud of their achievements. “
main research
This main study found that 17 patients were blinded and randomized to receive a placebo dose for one month and six months or Herantis Pharma CDNF for six months during the same period. Since then, 15 patients have participated in another 6-month study, and all participants have received CDNF. Patients who completed the two studies received a total of 12 infusions, which were performed in an outpatient setting.
The safety, performance and tolerability of the nerve injection system and CDNF, as well as the accuracy of the operation were evaluated. Among other indicators, the potential efficacy of the drug was scored according to the Unified Parkinson’s Disease Score Scale (UPDRS) exercise score.
The clinical study was funded by the European Union’s research and innovation program Horizon 2020. Preliminary results show “promising”, the device can be placed predictably and accurately, and the device has significant efficacy and safety.
3D printingNerve implant
In use3D printingIn the process of creating neural implants, Renishaw was not alone. Researchers and engineers at the Massachusetts Institute of Technology (MIT) explored the use of3D printingTo use conductive polymer liquid materials to develop soft and flexible brain electrodes.
In 2019, researchers at Carnegie Mellon University announced a study using 3D nanoparticle printing to create high-density neural probes to record neurological data. At the same time, Qrons, a New York-based biotechnology startup, announced an intellectual property (IP) agreement with Dartmouth College to develop 3D printable implants to treat penetrating or traumatic brain injuries.
China3D printingNet compile article!
(Editor in charge: admin)


0 Comments for “Renishaw completes clinical trial of nerve infection drug delivery system for Parkinson’s disease”