On January 10, 2020, developed by doctors at the Cleveland Clinic in Ohio, USA3D printingThe tracheal stent has been approved by the U.S. Food and Drug Administration (FDA), the hospital is a non-profit multidisciplinary academic medical center in Ohio.
Created by Cleveland Clinic physician Tom Gildea, MD3D printingSilicone stents can help patients with severe breathing disorders keep their airways open. Such breathing disorders can be caused by causes such as tumors, inflammation, trauma or other masses.Before obtaining FDA approval, doctors carried out clinical trials on some patients who could not be cured by conventional means, and3D printingThe stent is implanted in the patient.
3D printingThe tracheal stent is custom-designed based on the patient’s CT scan data and proprietary 3D visualization software, which overcomes the common problems of standard stents, such as possible tracheal complications for patients.
Breathing is something that many people take for granted, but for many such patients, every breath can be a struggle. Dr. Gildea, head of the bronchoscopy department at the Cleveland Clinic, said: “We are very pleased to bring this technology to more patients across the country, and thank the patients and donors who have worked with us to help apply this technology.”
 3D printingTracheal stent” alt=” 3D printingTrachea support” width=”620″ height=”620″ />
3D printingTracheal stent” alt=” 3D printingTrachea support” width=”620″ height=”620″ />
3D printingTracheal stent. The picture comes from the Cleveland Clinic.
3D printingAdvantages of tracheal stents
It is estimated that by 2020, approximately 30,000 tracheal stents will be implanted in the United States. Standard tracheal stents are designed with limited sizes and shapes and are usually best suited for individuals with larger trachea. However, there are often problems for different patients, because no two patients have the same anatomical structure, so it is difficult to obtain a perfect fit, especially for those patients with complex conditions. A stent that is incorrectly installed in the patient’s body may be kinked and bent, and may cause tracheal complications, such as the growth of new tissue, mucus impingement, and tissue death.
Dr. Gildea proposed that based on patient-specific data3D printingThe idea of tracheal stents, so as to personalize the anatomy of each patient. Dr. Gildea and his engineering team used CT scans and proprietary 3D visualization software to design the stent, and then based on the design3D printingMold, and then inject medical grade silicone into the mold to get the final scaffold.
In addition to achieving perfect fit, the use of patient-specific silicone stents also has the potential advantage of being more tolerable than traditional silicone stents. Due to the physical discomfort of some patients, spare stents may need to be replaced or cleaned frequently.
However, studies have shown that patient-specific stents can last for an average of one year, while standard tracheal stents only last for 90 days. In addition, patient-specific stents have shown shorter operation times and improved patient-reported symptoms. This can prove beneficial because it reduces the need for bracket replacement and modification, which are common problems with standard counterparts.
Provide patient-specific products to provide a personalized healthcare experience
use3D printingThe manufactured products for patients have brought many benefits to the entire medical sector. In addition to providing patients with a personalized experience, patient-specific products can also better inform medical professionals and prepare them for surgery.Therefore, as hospitals continue to adopt3D printingTechnology to provide personalized medical care, and this field is constantly evolving.For example, the Puget Sound Health Care System of Veterans Affairs (VA) recently announced a two-year collaboration with the University of Washington School of Medicine (UW) to create patient-specific3D printingModel to help treat mitral valve disease, complex heart abnormalities.Recently, GE Healthcare and Formlabs announced a collaboration to make it easier for clinicians to extract imaging data3D printingPatient-specific anatomical model.
Therefore, including tracheal stents3D printingThe patient-specific product was named one of the top ten innovations at the 2018 Cleveland Clinic Annual Medical Innovation Summit. Dr. Gildea also received the 2018 Inventor of the Year Reception hosted by the Cleveland Clinic Innovation of the American Medical Device Outstanding Innovation Award.
As personalized medical devices usually appear in the form of plastic surgery, the Cleveland Clinic subsidiary, which focuses on plastic surgery, promotes3D printingThe development and FDA approval of the patient-specific stent.In order to manufacture and provide3D printingBracket, a new subsidiary named VisionAir Solutions will be established, and the company will also work to integrate other3D printingThe equipment is brought to interventional pulmonologists and patients who need them. By the end of 2020, VisionAir Solutions plans to begin providing personalized stents to patients in medical institutions across the country in a controlled manner.
other3D printingTracheal progression
compared to3D printingTracheal stents, researchers in Japan and South Korea have achieved direct3D printingtrachea.
In 2018,
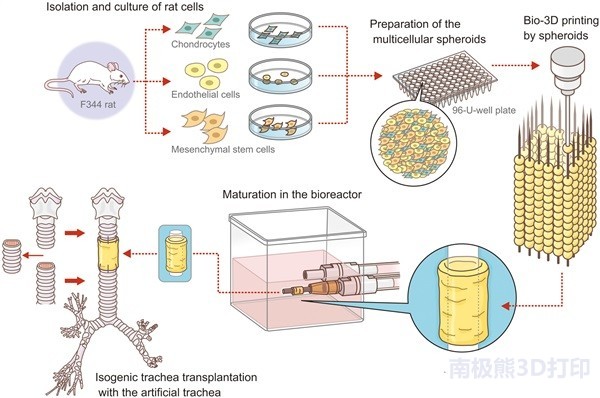
Researchers from Saga University and Nagasaki University in Japan use Cyfuse Biomedical Regenova 3D bioprintingStentless tracheal regeneration
. Tracheal transplantation in 9 rats was successful.
In 2019,
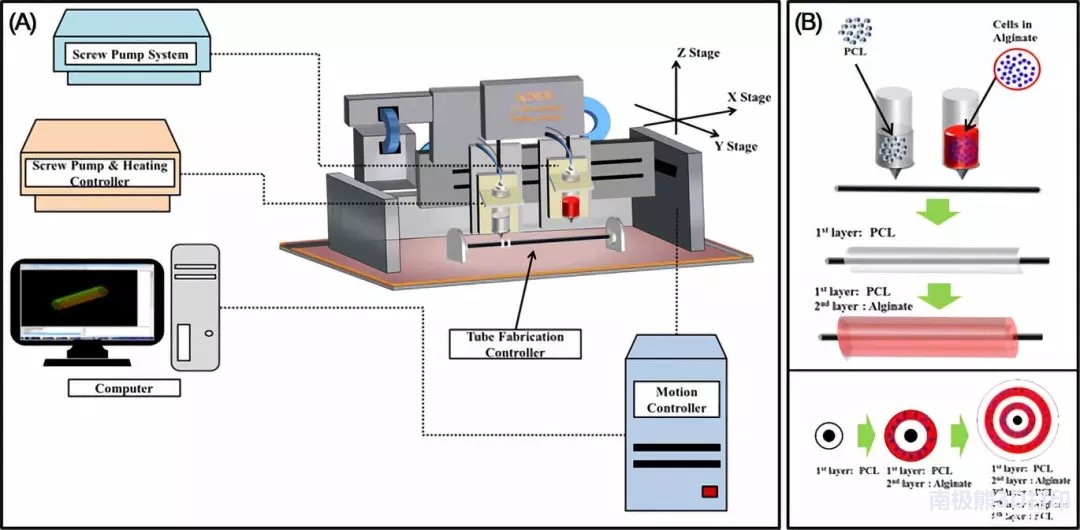
Researchers in South Korea announced their research results in a paper entitled “Trachea and Autologous Epithelial Cells and Cartilage Cells”
. The team of scientists detailed their method of printing artificial trachea using a mixture of polycaprolactone and hydrogel, nasal epithelial cells and auricular cartilage cells.
After using these materials and tissues to bioprint artificial trachea, they transplanted them into 15 rabbits, of which 6 were control groups. The goal is to find a way to overcome tracheal problems caused by tumors, the most common of which are adenoid cystic carcinoma and squamous cell carcinoma. In the past, there were great challenges in creating viable, correct, and capable of producing a new type of epithelial cell. With the occurrence of infection, some problems have arisen, and these problems have become diseases, have migrated, or have encountered obstacles.
In 2019,
The Jesus Children’s Hospital in Rome, Italy, recently performed operations for bronchomalacia and severe bronchial wall atrophy on a 5-year-old infant
, The hospital first adopted3D printingThe bronchial implantation operation was successful and helped the patient resume normal breathing.Experts say that implementing for patients3D printingBronchial implant surgery is the first in Italy and Europe.
Compiled from: 3dprintingindustry
(Editor in charge: admin)


0 Comments for “The 3D printing mold customized a special silicone tracheal stent for the patient, which was approved by the US FDA”