The rapidly growing novel coronavirus pneumonia epidemic in the United States has also caused a shortage of medical resources, among which throat swabs needed to detect the virus are in particularly high demand.At this moment3D printingTechnology plays a role, Formlabs partners with medical institutions to utilize light curing3D printingTechnology mass production of throat swabs.
 3D printingTechnology for mass production of throat swabs” alt=”3D printingTechnical mass production of throat swabs” width=”620″ height=”339″ />
3D printingTechnology for mass production of throat swabs” alt=”3D printingTechnical mass production of throat swabs” width=”620″ height=”339″ />3D printingTechnology for mass production of throat swabs
Nasopharyngeal (NP) swabs, commonly used to test for influenza and other respiratory infections, are required across the United States to collect COVID-19 test samples. The current supply chain shortage is so severe that clinicians are looking for ways to design and test new types of swabs.

A small brush at the front collects nasopharyngeal samples and sends them for inspection
NP pharyngeal swabs are flexible rods with bristle ends that can be inserted through the nose to the back of the nasal cavity and swept around to collect adherent samples to be tested, and the swab is then placed into a vial containing culture medium.in design3D printingThe throat swab is set as a breaking point seven or eight centimeters back from the brush, so as to retain the sample site at the front and just enough to seal the vial for easy transportation to the laboratory for subsequent testing.
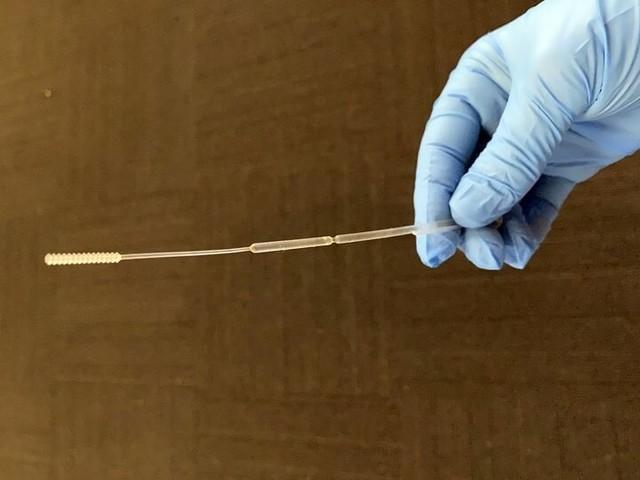
Front brush and rear break point
Formlabs teamed up with physicians at USF Health and Northwell Health to create a new swab design to improve efficiency and deliver as many swabs as possible to the health department.whole cotton swab3D printingAs one, and continuously improve the design.
Formlabs uses biocompatible, autoclavable Surgical Guide resin material,3D printingHundreds of test swab samples were taken. The samples have passed various USF Health tests and received emergency approval from the IRB for regulatory compliance, infectious diseases and virology.
These3D printingSwabs are Class I medical devices exempt from the premarket notification requirement, requiring the manufacturer to register and list the product. Formlabs will manufacture the swabs at its US FDA registered, ISO 13485 certified facility.

Ventilator multi-port valve
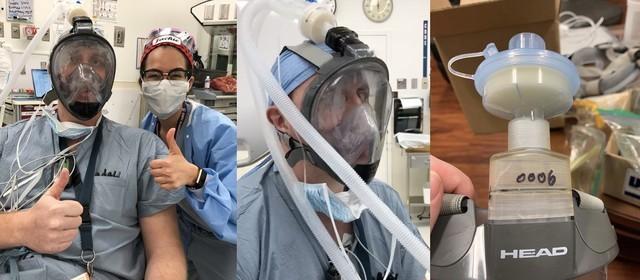
mask adapter
In addition, Formlabs also3D printingMedical equipment such as mask adapters and ventilator multi-port valves are used for clinical diagnosis and treatment of the new coronavirus.
(responsible editor: admin)


0 Comments for “U.S. 3D printed throat swabs quickly replenish scarce medical resources”