The proposal of “Made in China 2025” indicates that the transformation and upgrading of my country’s medical device industry is accelerating, and the research and development trend is also moving closer to the world. At present, domestic medical device products are still concentrated in the low-end varieties, and the high-end implantable devices are in a critical period of competition from imitating innovation to partially replacing imports. The domestic medical device industry is gradually increasing the dimension of product innovation. Because high-end implantable medical devices are very sophisticated, the corresponding manufacturing and processing technology requirements are getting higher and higher, and traditional processing methods are difficult to meet the requirements for rapid innovation of implanted medical devices. , Looking for innovative precision processing methods has become an urgent need for industry innovation.
business background
Medical devices refer to instruments, equipment, appliances, in vitro diagnostic reagents and calibrators, materials, and other similar or related items used directly or indirectly on the human body, including the required computer software. Among the many subdivisions of medical devices, based on multi-dimensional analysis of market size and growth rate, competitive landscape, and policy support, the introduction of implantable devices is one of the most potential subdivisions, and policy support is also the promotion of mediation. An important factor in the rapid development of implantable devices. In recent years, in the field of innovative medical devices, my country has issued a number of powerful policies to promote the innovation capability and industrialization level of local medical devices, and the introduction of implantable devices has clearly appeared in such areas as the “13th Five-Year Plan” outline and national technological innovation. Plans and other documents are within the scope of encouragement and support.
With the improvement of the economic and living standards of Chinese residents, the awareness of medical care has gradually strengthened, so the demand for medical equipment products is also rising. Although the market capacity of my country’s medical device industry is expanding rapidly, due to the backwardness of related basic science and manufacturing technology, domestic medical device products are still concentrated in low-end products, and high-end medical devices mainly rely on imports. The proposal of “Made in China 2025” indicates that the transformation and upgrading of my country’s medical device industry is accelerating, and the research and development trend is also moving closer to the world. In the future, with the support of national policies, expanding market demand, the acceleration of China’s population aging, and the technological development and industrial upgrading of the medical device industry, medical devices are expected to continue to maintain a good momentum of rapid growth and realize the transition from the low-end market to the high-end market. The vision of market import substitution.
In recent years, my country’s medical device industry has continued to develop rapidly. The growth rate of the high-value medical consumables market ranks among the top in the industry, with an average annual growth rate of over 20%. The largest market segment of consumables has always attracted the attention of the industry. Media implantation devices are currently in a critical period of reshaping the competitive landscape from imitating innovation to import substitution, and even leading the world. Domestic medical device companies are also gradually increasing the intensity of product innovation and creation. Because implanted medical devices are very sophisticated, the requirements for corresponding manufacturing and processing technologies are also getting higher and higher, and traditional processing methods are difficult to keep up with the rapid speed of implanted medical devices. The pace of innovation and the search for innovative precision machining methods have become their urgent needs.
market Overview
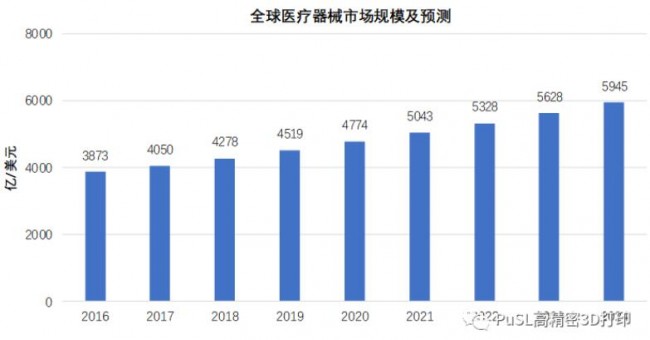
According to the medical device market released by iiMedia Consulting, globally, the global medical device market exceeded US$400 billion in 2017, and the global medical device market was US$427.8 billion in 2018. It is expected to be close to US$600 billion by 2024. The compound growth rate in 2024 is 5.6%. China has become the fourth largest medical device market after the United States, Western Europe, and Japan. The overall market size of China’s medical devices has grown from 255.6 billion yuan in 2014 to 530.4 billion yuan in 2018, with an average annual growth rate of around 20%, and both operating income and net profit have maintained a rapid growth trend, which is the gold medal for the development of the medical device industry. In the period, it is estimated that by 2022, the scale of China’s medical device market will exceed 900 billion yuan.
At this stage, the basic composition of my country’s medical device market is that high-end products account for 25%, and low-end products account for 75%. The basic composition of medical device products in the international medical device market is that high-end products generally account for 55%. Low-end products accounted for 45%. And in the high-end product market that accounts for 25% of my country’s medical equipment, 70% is occupied by foreign capital. This 70% of foreign- Medical device companies mainly produce low-end products. In the high-end medical device market such as implantable devices, due to the high technical content and added value of its products, it is not easy to develop and manufacture, technical level restrictions and resource monopolies in developed countries, which account for only about 30%. High-end products in the industry still rely mainly on imports. The high-end product market is mostly occupied by foreign companies such as Medtronic, Stryker, Abbott, GE Healthcare and Boston Scientific.
Based on multi-dimensional analysis of market size and growth rate, competition pattern, policy support, etc., implantable devices are one of the most potential subdivisions of medical devices. In terms of market size and growth rate, according to a report released by Evaluate MedTech, by 2024, in the global $600 billion medical device market, cardiovascular, neurological, orthopedic, dental, and ophthalmic equipment closely related to implantable devices will be included in the global medical device market. All will be in the stage of large market and high growth. Insufficient investment in innovation, poor technology research and development capabilities, and unclear business models have become obstacles to the future development of domestic enterprises in the implantable medical device industry. Benefiting from market demand and policy guidance, China’s implantable interventional medical device companies will increase and focus on technological innovation in the next 10 years, thereby releasing the innovative dimension of implantable medical devices.
High Precision3D printingApplication in the implantable medical device industry
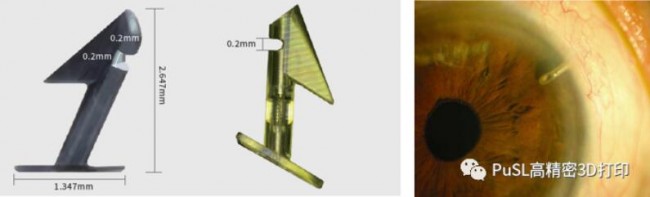
In recent years, the aging of the population has intensified, and unreasonable eye use habits have increased. The incidence of eye diseases in my country is high, showing a trend of early age, rapid progress, and deep severity. Although the number of ophthalmology patients has grown rapidly, the market penetration rate of ophthalmology products in my country is still relatively low due to the limitation of technology and economic development. Ophthalmology is a highly sophisticated discipline with high industry thresholds, especially in the field of high-value medical consumables, which requires high levels of refinement of materials and technologies. Therefore, the current global market is mainly concentrated in the hands of several large international medical equipment companies, and the competition is relatively high. Low. The picture below is an implantable guide nail that Shenzhen Morfang cooperated with a top domestic eye hospital. The product size is 2.647*1.347mm. Shenzhen Morfang S140 printing equipment can form nearly 2000 products at a time. There are very fine and small models in the model. And complex structure, which contains a spring and ball valve. Glaucoma is mainly caused by high intraocular pressure, which compresses the optic nerve. At present, this kind of guide nails in China are formed by machining titanium alloy, and the price is 3500 yuan each. Due to the limitation of the freedom of machining and forming, the structure of traditional guide nails is relatively simple. Drainage nails with micro springs can stably release intraocular pressure and improve the implantation experience and patients of glaucoma patients. With its breakthrough precision manufacturing capabilities, Mofang provides a revolutionary minimally invasive treatment plan for glaucoma treatment.
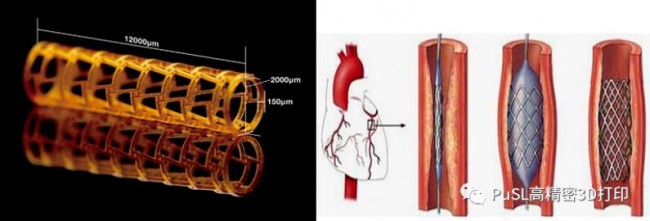
In 2016, nearly 180 million people died of cardiovascular disease globally, accounting for 31% of all-cause deaths. According to the “Report on Cardiovascular Diseases in China”, the prevalence and mortality of cardiovascular diseases in my country are rising, and the number of patients is about 290 million. The early treatment of cardiovascular diseases mainly relies on drugs, while the middle and late stages require surgical intervention, such as blood vessels. Stents, heart valves, pacemakers and other equipment. Vascular stents are the most widely used products in cardiovascular implantation devices, mainly used to treat vascular occlusion or stenosis. The current mainstream stent is a drug-loaded metal stent. However, due to the nondegradability of metal can cause vascular inflammation, a new generation of drug-loaded polymer degradable stents will become the mainstream direction of future research. The picture below shows the polymer vascular stent processed by our Shenzhen Morfang S140 equipment with biocompatible materials. It is 12mm high, 2mm diameter, and 0.15mm rod diameter. The printed stent has good retractability.
As the world’s aging trend deepens and environmental problems become increasingly severe, the incidence of diseases such as the digestive tract and respiratory tract continues to increase, and the demand for endoscopy is also increasing. Medical endoscopy technology has attracted wide attention from the medical community due to its high diagnosis and treatment accuracy, small trauma, low infection, fast postoperative recovery and almost no scars. It is also one of the fastest-growing products in the global medical device industry. With the advent of miniaturization and customization, product structures are getting smaller and thinner, and endoscopy companies are all committed to finding matching precision processing methods. For precision endoscope end seats with a wall thickness of less than 0.15mm, traditional processing methods such as CNC and open-mold injection molding are difficult to form, especially for thin-walled parts with large aspect ratios. The wall thickness of the round tube in the end seat of the endoscope in the picture below is 70 microns, the tube diameter is 1mm, the height is 4mm, and the accuracy is ±10~25 microns. It is difficult to process such a limit by CNC and mold injection. According to the structure, the P140 equipment of Shenzhen Morfang Company can process high-quality and qualified products in about two hours and can be delivered within one day as soon as possible.

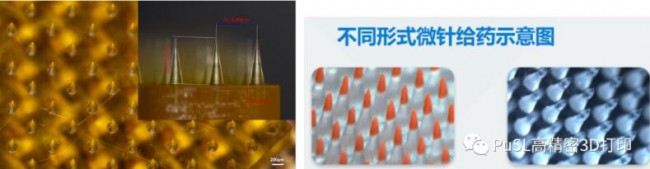
At present, China’s medical aesthetics service consumer groups are relatively younger, creating conditions for forming medical aesthetics consumption habits and creating a longer consumption cycle. According to a Deloitte report, the size of China’s medical aesthetics market was 176 billion in 2017. According to ISAPS forecasts, by 2020, China’s medical aesthetics market is expected to reach 315 billion yuan. As the Chinese people increasingly respect the medical beauty industry, the medical beauty industry will become a hot industry in the future. The picture below is a cosmetic microneedle with an array structure processed by Shenzhen Mofang’s S130 equipment. The bottom of the microneedle is 0.198mm straight and 0.572mm high. The width of the tip is only 0.006mm. The surface of the processed microneedle is smooth and the details of the tip are clearer. . The microneedle printing material belongs to the acrylic polymer type of microneedles. Usually, the polymer is used to print the needle tip shape male mold, and the actual required medical polymer needle tip structure is formed through the second inversion mold, for example, the dissolved microneedle is formed.Through Morfang Precision3D printingThe equipment can well solve the limitations of traditional microneedle processing, and has a very significant and positive impact on promoting the development of the medical aesthetics industry.
(Editor in charge: admin)


0 Comments for “Unleash the innovative dimension of implantable medical devices with the help of high-precision 3D printing technology”