As of March 30, 2020, the number of confirmed cases of new coronary pneumonia in the United States has exceeded 140,000, the highest in the world; at the same time, the total number of confirmed cases in the world has exceeded 700,000. All kinds of related protective medical supplies are not enough.

△Used by college students from two medical departments3D printingmachine DIY mask
On March 26, 2020, the U.S. Food and Drug Administration (FDA) issued the “Regulations on Medical Devices, Accessories, Parts During the COVID-19 Pandemic”3D printingFrequently Asked Questions”
want to3D printingMasks, gowns and other personal protective equipment (PPE) to fight the coronavirus pandemic?You might want to see what the FDA has to say about this
Regulations
.
In response to COVID-19, the FDA continues to employ innovative and flexible approaches to address the supply of critical medical products. During the COVID-19 pandemic, demand for certain medical equipment, including personal protective equipment (PPE), may exceed the supply capacity of healthcare facilities due to a surge in global demand for supplies coupled with massive supply chain disruptions.We recognize that during the COVID-19 pandemic, the public may seek to use3D printingto meet the production of certain products.As part of efforts to protect the public as much as possible, the FDA is3D printingEntities of equipment, accessories, assemblies and/or parts provide answers to frequently asked questions.
Q: FDA’s3D printingWhat are the general recommendations for medical devices?
A: The FDA has issued guidance on technical considerations for additively manufactured medical devices in December 2017
.This guidance provides an overview of FDA’s process validation from the device stage, through the finished device, to the approved3D printingequipment recommendations.
ask:3D printingCan it be used to make protective clothing, masks, respirators and other types of personal protective equipment (PPE)?
A: PPE includes protective clothing, coveralls, gloves, masks, goggles, masks, and respirators, or other equipment designed to protect the wearer from injury or the spread of infection or disease.Although you can use3D printingto make some PPE, but effectively solve some technical challenges. For example,3D printingThe PPE may provide a physical barrier, but3D printingThe PPE is unlikely to provide the same liquid barrier and air filtration protection as FDA-approved surgical masks and N95 respirators.US Centers for Disease Control and Prevention CDC
How to optimize masks
give suggestions.
ask:3D printingcan it achieve the same fluid barrier protection and air filtration as FDA-approved surgical masks and N95 respirators?
answer:3D printingThe mask shell may look like traditional PPE. However, they may not provide the same level of barrier protection, fluid resistance, filtration and infection control. The CDC has recommendations on how to optimize masks.

△3D printingface mask
Q: If you use3D printingWhat should healthcare workers do?
A: Health care providers want this
- an examination3D printingWhether the mask seal is leaking.
- Confirm that their material has a breath-filtering effect.
- Take extra care in surgical environments where a protective fluid barrier and flammability are required.
- Recognize that face coverings may not provide adequate air filtration and be prepared to prevent the spread of infectious agents.
- Safely dispose of infectious materials and sanitize any parts that are reused.
Q: Is it possible3D printingAccessories, components or parts for medical equipment?
A: In practice, genuine parts or parts with the same specifications, dimensions and performance (if available) should be used.Although it is possible to use3D printingto print certain accessories, assemblies and parts, but some complex products (e.g. working pumps, electronics) are not easily3D printing.Where possible, it may be helpful to use the design of the original part; use any3D printingBefore using the product, it needs to be verified that it is suitable and working properly.encourage engagement3D printingThe units cooperate with relevant medical device manufacturers.

△ Institute CIIRC CVUT and HP design and3D printingmasks
Q: Can the entire medical device be3D printing?
A: Although FDA understands3D printingGreater equipment availability may be available during the COVID-19 public health emergency, but some equipment may be more suitable3D printing. FDA is willing to discuss these and other issues with manufacturers and agencies.The person concerned should send an email to
[email protected]
for more information.
Q: How can FDA alleviate shortages of PPE and components, parts, and accessories?
A: We recognize that when regular products are not available, some people are considering3D printingor buy3D printingequipment. FDA is working closely with government, industry and healthcare sector stakeholders on the broader public health emergency. The FDA recently granted Emergency Use Authorization (EUA) for ventilators, ventilator circuit connectors, and ventilator accessories, which may include an EUA for emergency use of a medical device in an emergency. multiplexed ventilator3D printingLine connector. FDA also has public-private partnerships with the Department of Veterans Affairs (VA) Innovation Ecosystem, the U.S., and the National Institutes of Health (NIH)3D printingThe exchange, which is a resource of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health.
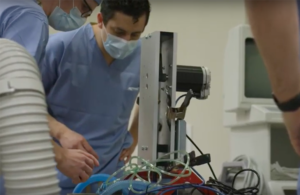
△DIY ventilator, some parts are also3D printingof
Antarctic bears have noticed that with the growing demand for such devices and the popularity of EUAs, some research teams are starting to develop makeshift “DIY” ventilators, such as engineers at the University of Minnesota, who have launched one that costs as little as $150 the ventilator. However, although the FDA’s guidance on EUA includes use as a substitute for a medical device, there is no clear information on whether this “Coventor” device can be authorized.
(responsible editor: admin)


0 Comments for “US FDA: Prevention and control of new coronavirus, FAQs for 3D printing medical equipment/accessories/parts”