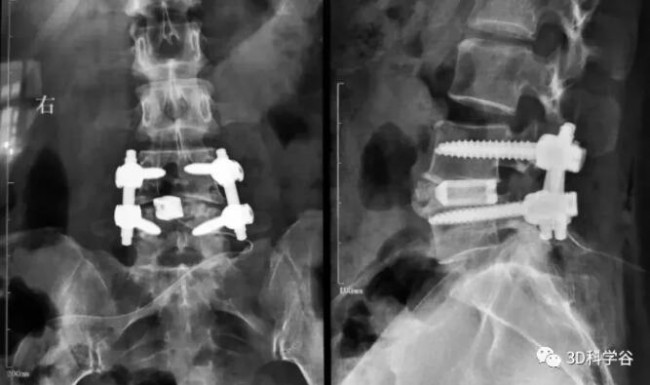
In September 2021, Zhongnuo Hengkang Additive Manufacturing-3D printing The “Intervertebral Fusion Cage” was approved by the State Drug Administration for marketing. The product was developed and clinically validated by Zhongnuo Hengkang for 6 years, and completed the marketing registration approval for innovative products with independent intellectual property rights.This product will further promote our country3D printingThe commercialization of titanium alloy spinal implants is conducive to its clinical application in degenerative spinal diseases.
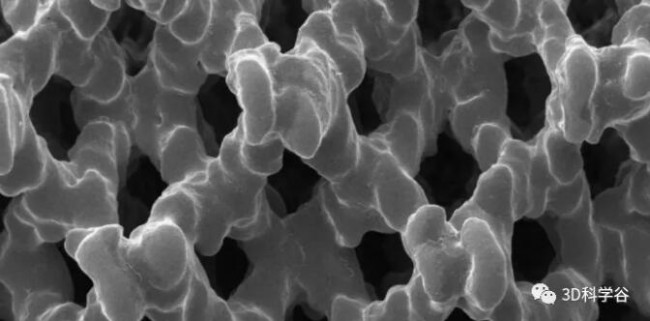
This approved3D printingIntervertebral fusion cage: The pore diameter is 300-800μm, and the porosity is 70-80%. It is the first domestic fusion cage product without bone grafting window using additive manufacturing technology. It has good biocompatibility and elasticity like cancellous bone. Modulus, surface microporous structure provides good initial stability.
At the same time, the product has multiple models and specifications that can meet a variety of surgical needs. The use of the product conforms to the operating habits of the fusion cage, allowing clinicians to quickly adapt to the product’s intraoperative implantation and surgical promotion.
This time3D printingThe issuance of the registration certificate for the bone graftless interbody fusion cage will be further promoted3D printingThe application of technology in my country’s medical field, especially the development of spinal surgery.Zhongnuo Hengkang will continue to play further in the medical field through the “medical-industrial interaction” research and development model3D printingThe advantages of technology provide more innovative, high-quality and efficient medical products and service systems for clinics and patients, and better benefit the society.
(Editor in charge: admin)



0 Comments for “Zhongnuo Hengkang’s 3D printing “intervertebral fusion cage” was approved by NMPA”